Subpleural sparing refers to a pattern of lung abnormality where the lung regions directly adjacent to the pleura are preserved, showing little or no involvement compared to the diseased lung parenchyma in other areas. It is commonly identified on high-resolution computed tomography (HRCT) and is a critical finding in the differential diagnosis of interstitial lung diseases (ILDs).
Causes
Subpleural sparing is typically associated with certain conditions, most notably:
Inflammation
- Non-Specific Interstitial Pneumonia (NSIP):
- Commonly shows subpleural sparing as a distinguishing feature from usual interstitial pneumonia (UIP), where the subpleural regions are heavily involved.

CXR performed 10 years prior t COVID infection and CXR and CT performed after COVID infection Ashley Davidoff MD The CommonVein.net

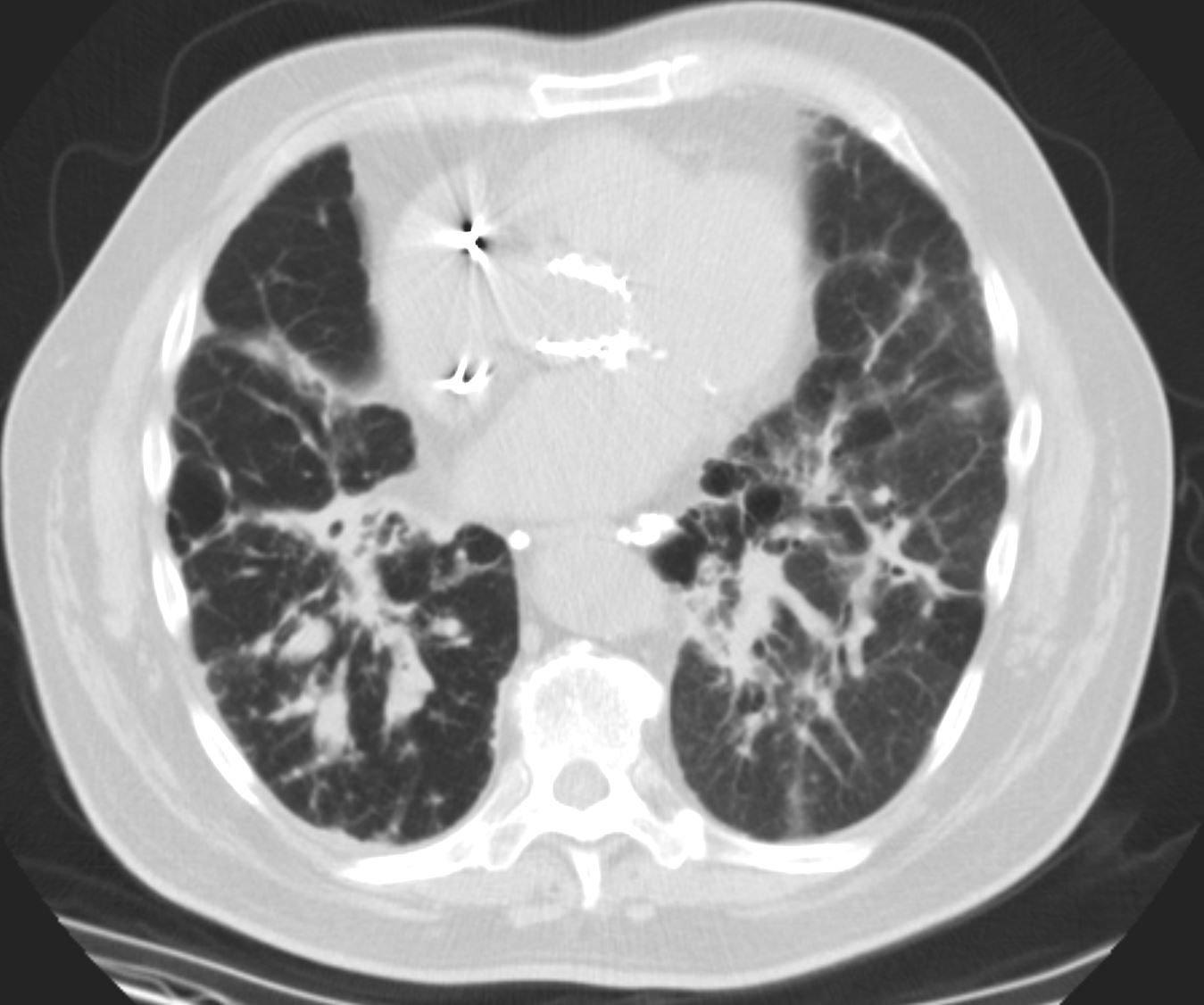
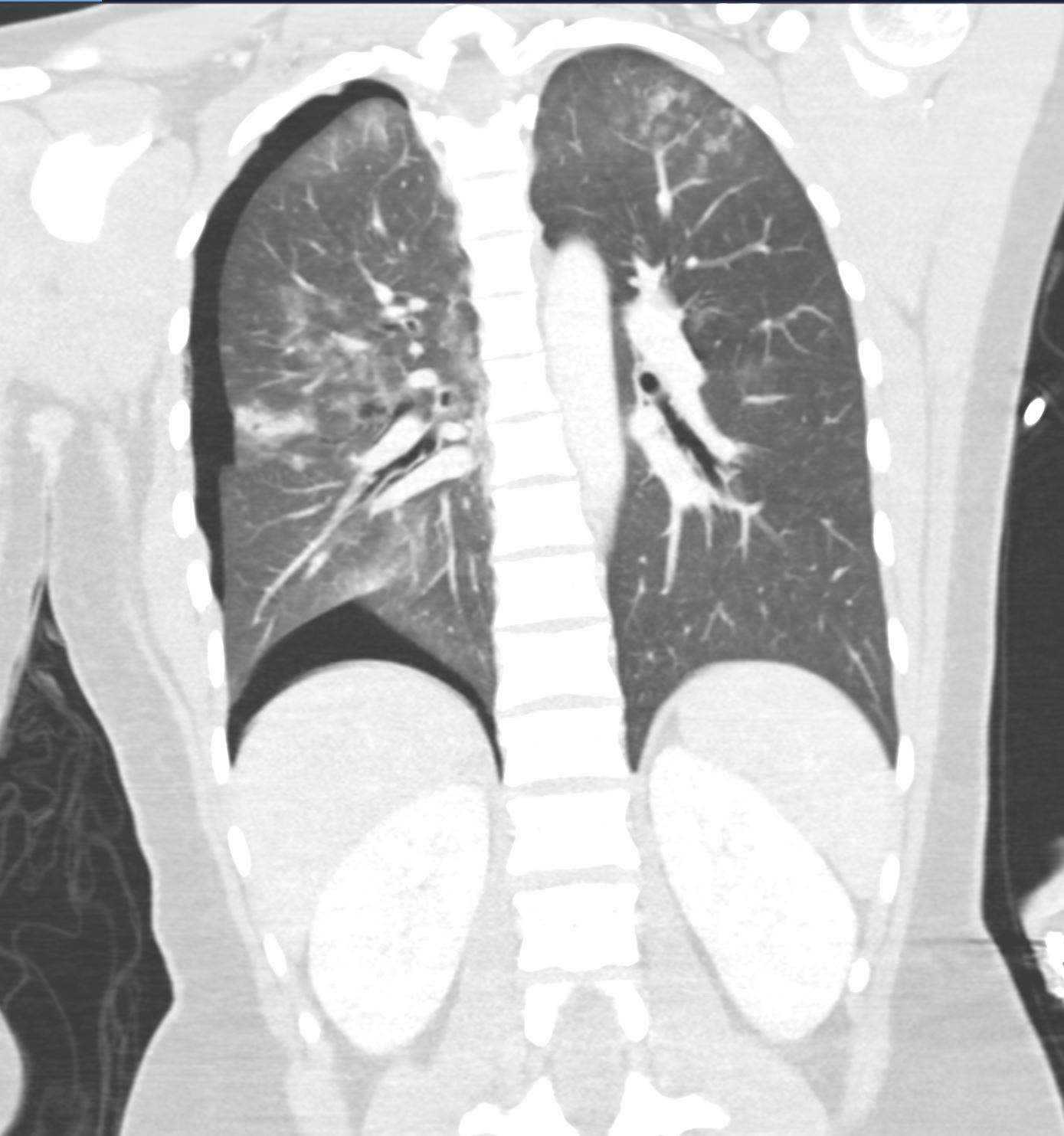
71-year-old female presents with a history of scleroderma, ILD, hypothyroidism and dcSSc
CT in the axial plane, shows extensive ground glass changes in the inferior lingula middle lobe and traction bronchiectasis in the middle lobe. The left lower lobe shows bronchovascular changes, and bronchiolectasis and there is bilateral subpleural sparing
The fissures show irregular thickening.
The lower image exemplifies the traction bronchiectasis
Ashley Davidoff MD TheCommonVein.net 196Lu 136610c
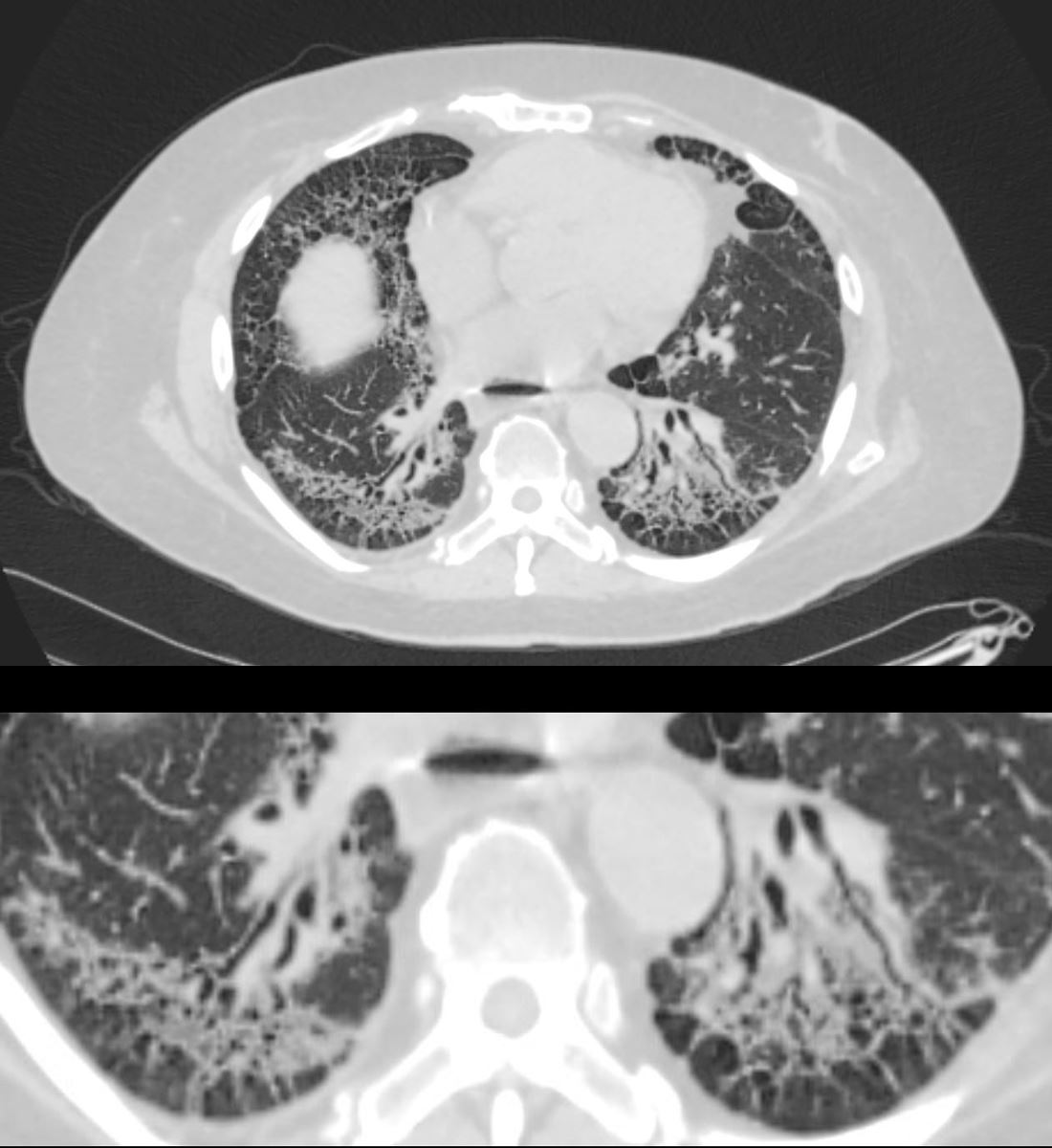
59-year-old male presents with history of scleroderma, Raynaud’s disease, and ILD
Upper Image
Axial CT shows bibasilar ground glass, bronchiectasis, and bronchiolectasis with volume loss and with crowding of the bronchovascular bundles posteriorly. There is subpleural sparing. Note air-fluid level in the distended esophagus.
The lower image focuses on the peripheral sparing. The spared secondary lobules have also undergone enlargement secondary to the fibrotic process
Ashley Davidoff MD TheCommonVein.net 110Lu 136598c01
- Connective Tissue Diseases:
- Especially in systemic sclerosis, dermatomyositis, and other autoimmune diseases with pulmonary involvement.
- Hypersensitivity Pneumonitis (HP):
- In chronic HP, subpleural sparing may be seen as part of a diffuse pattern of ground-glass opacities and fibrosis.
- Sarcoidosis:
- Advanced stages may show relative subpleural sparing despite other fibrotic or granulomatous changes in the lung parenchyma.

Ashley Davidoff TheCommonVein.net
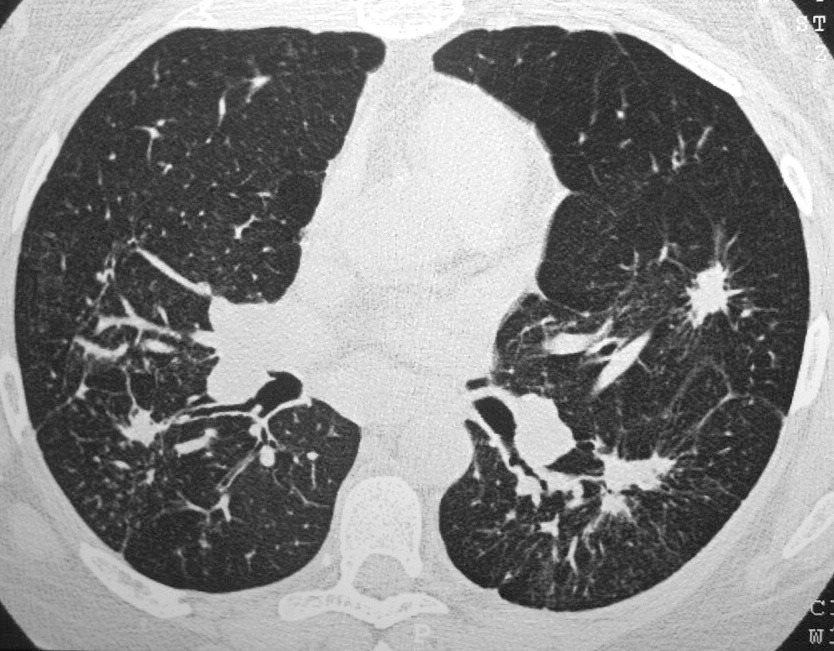
42 year old female with known history of sarcoidosis characterised by confluent granulomas, with spiculated nodules, retractile fibrosis and moderate adenopathy
Ashley Davidoff MD TheCommonVein.net 31828

SARCOIDOSIS, STAGE IV, PTX, ENCASEMENT
50-year-old male presents with history of Stage 4 sarcoidosis acute chest pain and dyspnea
The initial CXR shows a left sided pneumothorax, diffuse nodular pattern with confluent perihilar infiltrates and a left pleural effusion
A chest tube was placed and a chest CT showed confluent fibrotic masses in the hilar regions totally surrounding the bronchovascular bundles with encasement of the middle lobe artery. In addition, multiple lymphovascular micronodules are demonstrated. The pulmonary artery measures 32.7mm indicating pulmonary hypertension.
A CXR during this admission shows re-expansion of the pneumothorax. Left lung volume is reduced.
The patient presents 2 years later, again with progressive dyspnea and chest pain and CT PA shows encasement of the airways, right middle lobe pulmonary artery and left lower pulmonary vein by the fibrotic broncho vascular masses, and non-occlusive, subacute pulmonary embolus of the LPA. There are moderate bilateral pleural effusions, calcified lymph nodes, with ongoing pulmonary hypertension with right ventricular enlargement, right atrial enlargement, tricuspid regurgitation and pulmonary hypertension. At this time the patient is intubated.
He again presents 1 month after with chest pain and dyspnea. At this time, he has a tracheostomy. The scout frontal view shows persistent encasement of the left upper lobe bronchus and significant reduction on the volume of the left lung with elevated left hemidiaphragm.
CT PA has similar findings with a large right pleural effusion and unresolved large non occlusive thrombus in the left pulmonary artery.
Ashley Davidoff MD TheCommonVein.net 132195.8L
- Drug-Induced Lung Disease:
- Certain chemotherapeutic agents and other medications can cause interstitial changes with subpleural sparing.
- Other Conditions:
- Early organizing pneumonia or pulmonary involvement in vasculitis can also display subpleural sparing.
- Hemorrhage
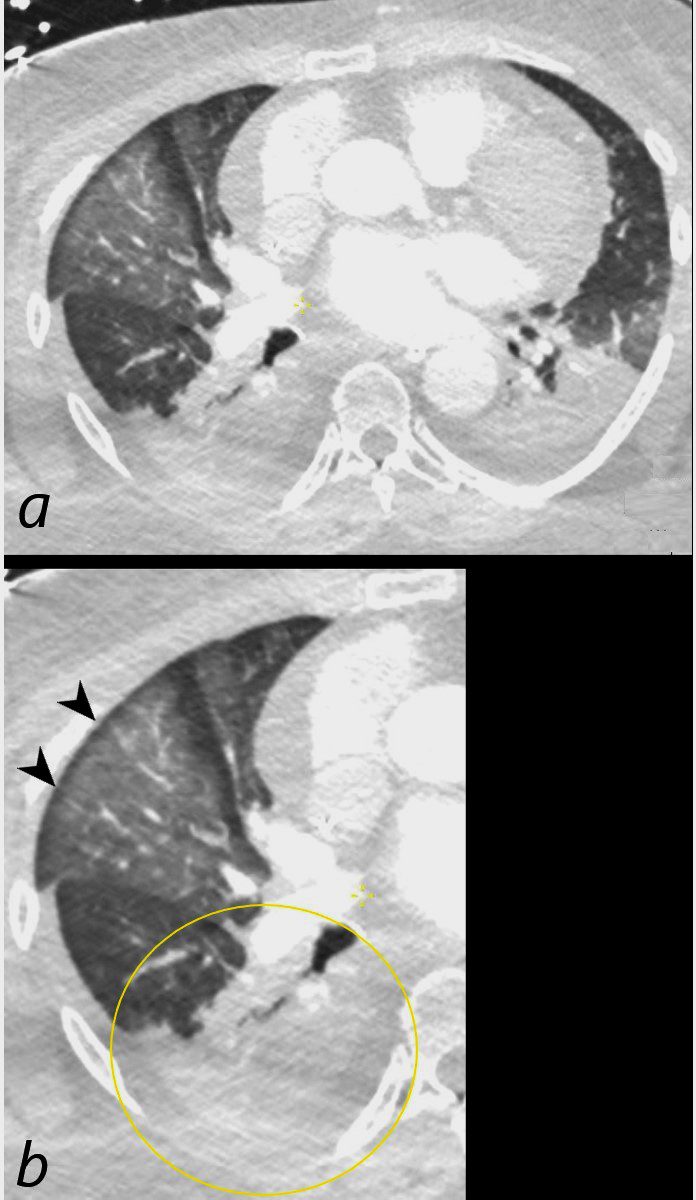
Axial CT scan at the level of the middle lobe of a 64-year-old female who arrested and required resuscitation is shown. Image a shows diffuse ground glass opacification in the lateral segment of the middle lobe with subpleural sparing (b, black arrowheads) most likely representing edema or hemorrhage. Bibasilar pneumonic infiltrates with bilateral effusions are present, exemplified and magnified in the right lower lobe (b, ringed in yellow)
Ashley Davidoff MD TheCommonVein.net 136192cL
24 year old female CT of the chest following blunt chest trauma shows a combination of findings including a small right sided pneumothorax, right sided contusion and embedded pneumatocele, and biapical ground glass changes. The left apical GGO also contains acinar shadows
Ashley Davidoff MD TheCommonVein.net137733
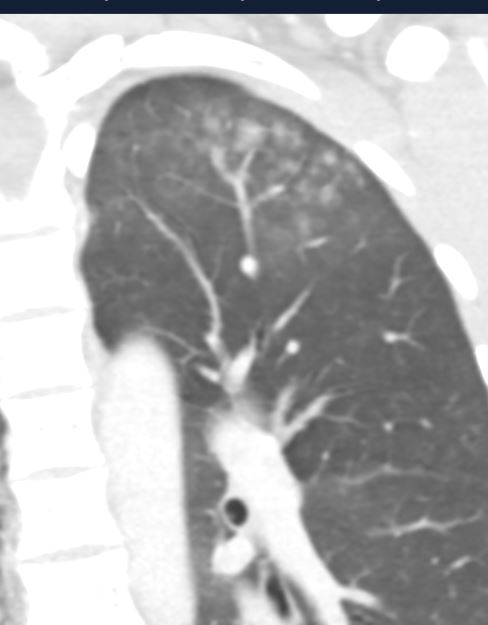
24 year old female CT of the chest following blunt chest trauma. The magnified view of the left apex reveals a GGO with ns acinar shadows and associated subpleural sparing consistent with parenchymal contusion and hemorrhage.
Ashley Davidoff MD TheCommonVein.net137735
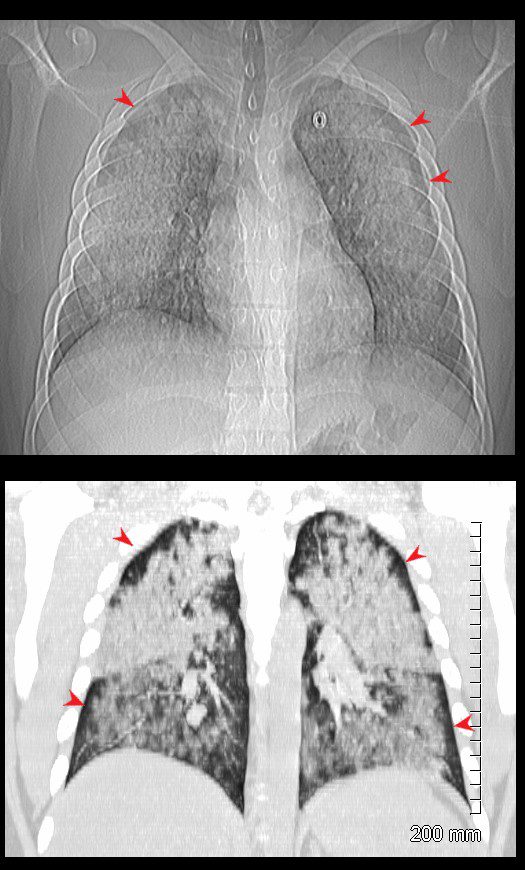
19 year old male previously well with history of hemoptysis, sweating, fevers, myalgias, arthritis over 3 weeks.
CT scan scout (above ) shows diffuse bilateral lobar infiltrates with subpleural sparing (red arrowheads)
Coronal CT shows bilateral symmetrical lobar nodular consolidations involving the upper and lower lobes. The upper lobes are more consolidative and the lower lobes have an acinar pattern. Subpleural sparing again noted (red arrowheads). These finding are consistent with acute pulmonary hemorrhage
Lab shows ANCA positivity, acute renal failure (creatinine 6) and renal biopsy showing crescentic glomerulonephritis. Treated with cyclophosphamide
Ashley Davidoff MD TheCommonVein.net 139195cL
CT Diagnosis
Subpleural sparing is best detected on high-resolution CT (HRCT) of the chest, which provides fine details of lung parenchyma. Key features to look for include:
- Appearance:
- Relative preservation of the lung tissue immediately adjacent to the pleura.
- Often seen as a “rim” of normal lung between the pleura and the diseased areas (e.g., ground-glass opacities, reticulations).
- Distribution:
- The sparing is usually uniform and circumferential but may vary depending on the underlying disease.
- Key CT Findings in Associated Conditions:
- NSIP: Bilateral ground-glass opacities with basal predominance, subpleural sparing, and minimal honeycombing.
- Hypersensitivity Pneumonitis: Patchy areas of ground-glass opacities with relative subpleural sparing.
- Sarcoidosis: Fibrotic changes in the midzones with relative sparing of the periphery.
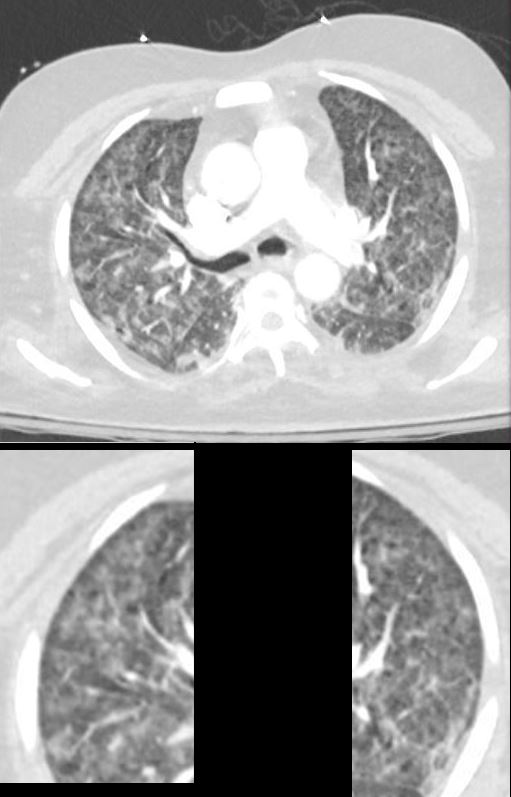
ARDS Sickle Cell Crisis
The CXR in this 26 year old male in sickle cell crisis shows diffuse and symmetrical ground glass changes with subpleural sparing
Ashley Davidoff TheCommonVein.net b11662-03
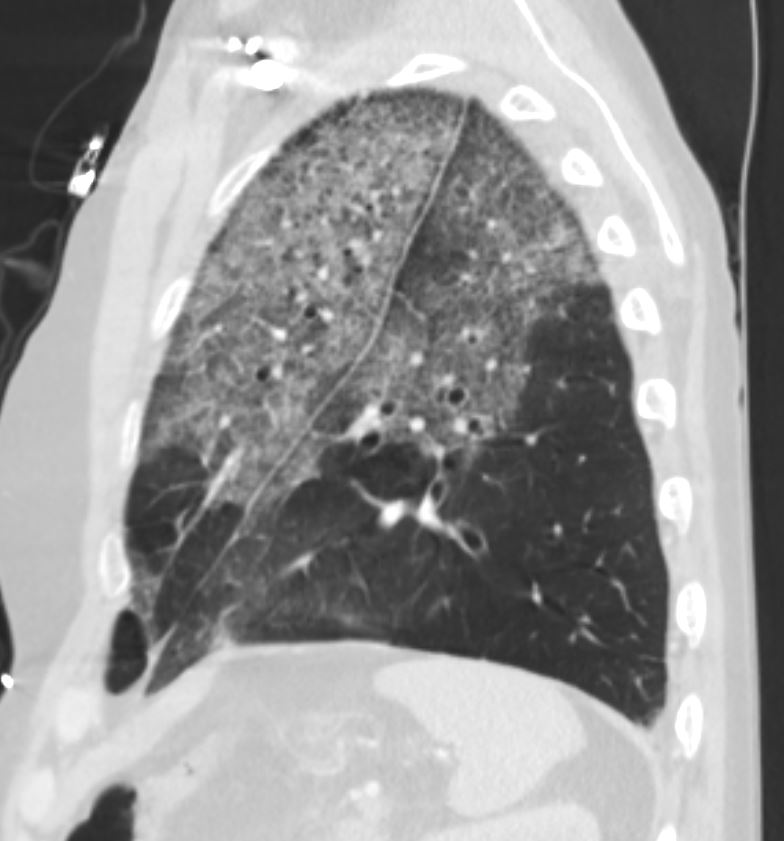
55-year-old male with substance use disorder presents with progressive and now more severe dyspnea. Sagittal CT through the left lung field shows ground glass changes in the upper mid and superior segment of the lower lobe. The fissures of the areas of involved lung are focally thickened. There is subpleural sparing and ?crazy paving? pattern suggested with thickened interlobular septa.
Progressive inhalational pneumonitis from smoking or cocaine inhalation was suspected. DIP and hypersensitivity pneumonitis remained in the differential diagnosis
(Crack Lung)
Ashley Davidoff MD TheCommonVein.net 251Lu 135943

Ashley Davidoff MD TheCommonVein.net crack-injury-009
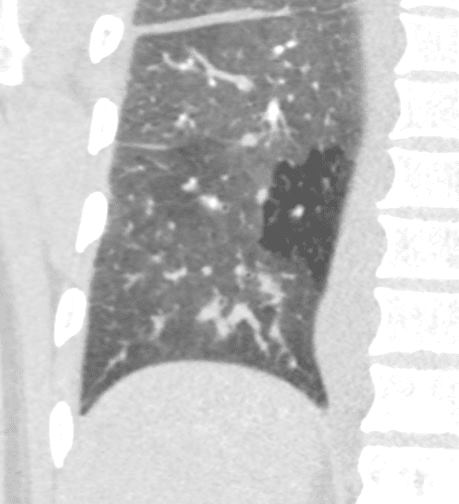
50 year old female with diabetes, chronic renal failure with congestive heart failure. CT in the coronal plane shows diffuse ground glass changes, Kerley B lines, edema in the fissure, and mosaic attenuation
Ashley Davidoff MD TheCommonvein.net 135779c 193Lu
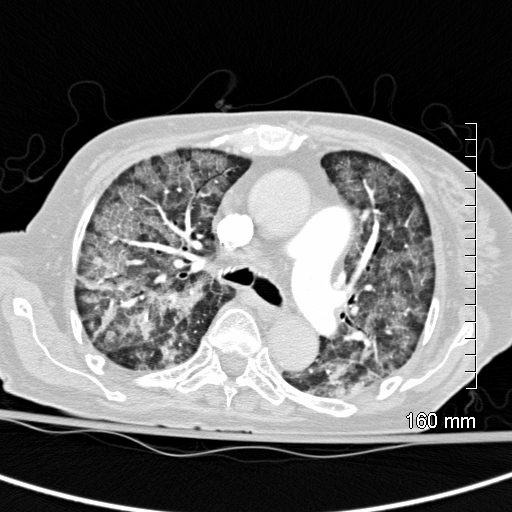
CT scan shows Diffuse ground glass pattern with thickening of the interlobular septa and manifesting as crazy paving pattern. Noted subpleural sparing bilaterally.
Ashley Davidoff MD TheCommonVein.net 131742
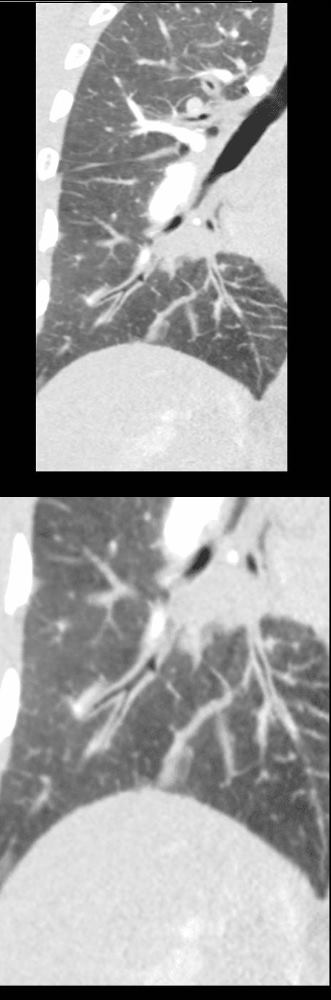
50 year old female with diabetes, chronic renal failure with congestive heart failure. CT in the coronal plane shows diffuse ground glass changes, with prominent peribronchial cuffing in the right upper lobe and and magnified in the lower image in the right lower lobe Prominent Kerley B lines are noted medially – a few laterally and there is with thickening of the right transverse fissure
Ashley Davidoff MD TheCommonvein.net 135777c 193Lu
Radiological Implications
- Differentiation of Interstitial Lung Diseases:
- Subpleural sparing is a critical diagnostic clue to differentiate NSIP from UIP, where the latter typically shows subpleural involvement and honeycombing.
- Management and Prognosis:
- Diseases associated with subpleural sparing, such as NSIP, often have a better prognosis and are more responsive to immunosuppressive therapy compared to UIP.
- Correct radiological identification ensures appropriate treatment pathways.
- Radiological Mimics:
- Awareness of conditions with subpleural sparing is crucial to avoid misdiagnosis, especially in distinguishing it from UIP or fibrotic hypersensitivity pneumonitis.
Conclusion
Subpleural sparing is a significant radiological finding with diagnostic and prognostic value in various interstitial lung diseases. Recognizing its pattern on HRCT aids in narrowing the differential diagnosis, guiding appropriate treatment, and improving patient outcomes.