Segmental Bronchi
Etymology
- Derived from the Latin bronchus, meaning “windpipe,” combined with “segmental,” indicating their role in supplying specific bronchopulmonary segments.
AKA and Abbreviation
- AKA: Tertiary bronchi
- Abbreviation: None commonly used
What is it
- The segmental bronchi are the third-order branches of the tracheobronchial tree, branching from the lobar bronchi.
- Each segmental bronchus supplies a specific bronchopulmonary segment.
- They are essential for directing airflow to distinct functional and anatomical lung regions.
Principles
Parts
- Right lung: 10 segmental bronchi corresponding to 10 bronchopulmonary segments:
- Upper lobe: Apical, posterior, and anterior segments.
- Middle lobe: Lateral and medial segments.
- Lower lobe: Superior, medial basal, anterior basal, lateral basal, and posterior basal segments.
- Left lung: Typically 8?10 segmental bronchi due to segmental fusion:
- Upper lobe: Apicoposterior (fused apical and posterior) and anterior segments.
- Lingula: Superior and inferior lingular segments.
- Lower lobe: Superior, anteromedial basal (fused anterior and medial basal), lateral basal, and posterior basal segments.
Size
- Diameter varies based on lung location and branching:
- Segmental bronchi are 2?4 mm in diameter proximally.
- Diameter decreases as they taper distally.
- Wall thickness
- If the diameter of the two walls added together is greater than 50% of the lumen it is considered thickened
Shape
- Tubular, with progressively thinner walls as branching continues.
- Reinforced by discontinuous cartilaginous plates.
Position
- Centrally located within each bronchopulmonary segment.
- Extend distally from lobar bronchi to supply subsegmental and alveolar airways.
Character
- Composed of:
- Cartilage plates for structural support.
- Smooth muscle and elastic fibers for flexibility.
- Mucosa lined with pseudostratified columnar epithelium.
Time
- Develop during embryonic lung branching (4th?6th weeks gestation).
- Continue to mature through childhood.
Blood Supply
- Supplied by bronchial arteries arising from the thoracic aorta.
Venous Drainage
- Drains into bronchial veins, which connect to the azygos and hemiazygos systems.
Lymphatic Drainage
- Drains into regional lymph nodes, including hilar and mediastinal nodes.
Nerve Supply
- Innervated by the vagus nerve (parasympathetic), sympathetic trunks, and pulmonary plexus.
Embryology
- Arise from the lung bud, derived from the ventral foregut during embryogenesis.
Histology
- Lined with ciliated pseudostratified columnar epithelium, with goblet cells for mucus production.
Physiology and Pathophysiology
- Direct airflow to distinct lung regions.
- Participate in mucociliary clearance.
- Pathological changes include inflammation, obstruction, or collapse, affecting ventilation and perfusion.
Applied Anatomy to Radiology
CXR (Chest X-ray)
- Indirect Visualization:
- Segmental bronchi are not directly visible due to their size.
- Pathologies inferred from:
- Segmental atelectasis: Wedge-shaped opacities corresponding to specific bronchopulmonary segments.
- Obstruction: Manifesting as distal consolidation or air trapping.
- Peribronchial cuffing: Indicates interstitial fluid or inflammation around the bronchi.
CT (Computed Tomography)
- Direct Visualization:
- Segmental bronchi are visible down to the subsegmental level.
- Pathologies identified include:
- Bronchial wall thickening: Seen in asthma or chronic bronchitis.
- Mucus plugging, air trapping, or peribronchial thickening further support the diagnosis.
- can be overestimated in:
- Expiratory-phase imaging (due to airway collapse).
- Patients with inadequate lung inflation during imaging.
- It is crucial to differentiate true wall thickening from:
- Peribronchial cuffing (fluid or inflammation in the interstitial tissue surrounding bronchi).
- Obstruction: Due to tumors, foreign bodies, or mucus plugs.
- Bronchiectasis: Dilated segmental bronchi with thickened walls.
- Infective changes: Centrilobular nodules or tree-in-bud appearance in tuberculosis or bronchiolitis.
- Bronchial wall thickening: Seen in asthma or chronic bronchitis.
- 3D Reconstruction and Virtual Bronchoscopy:
- Enables pre-procedural planning and assessment of airway patency.
MRI (Magnetic Resonance Imaging)
- Limited Role:
- MRI lacks sufficient resolution to visualize segmental bronchi directly.
- Advanced techniques like hyperpolarized gas MRI can indirectly evaluate airway function and ventilation.
Bronchography
- Historical Role:
- Contrast-based imaging of bronchi, now replaced by CT for safety and precision.
PET-CT
- Functional Imaging:
- Highlights increased metabolic activity in tumors or inflammation involving segmental bronchi.
- Valuable for staging lung cancers with bronchial involvement.
Endobronchial Ultrasound (EBUS)
- Guided Procedures:
- Allows real-time imaging and biopsy of structures near segmental bronchi, such as lymph nodes.
- Essential for diagnosing mediastinal pathology.
Pathological Implications
- Obstruction: Leads to distal atelectasis, air trapping, or infection.
- Bronchial wall thickening: Found in asthma, chronic bronchitis, or cystic fibrosis.
- Infections: Bronchopneumonia may localize to specific segments.
- Neoplasms: Bronchogenic carcinoma may arise from or involve segmental bronchi.
Key Points and Pearls
- Segmental bronchi are the functional units directing airflow to bronchopulmonary segments.
- Precise localization of pathology on imaging is critical for surgical or interventional planning.
- CT imaging is the modality of choice for segmental bronchial evaluation.
- If the diameter of the two walls added together is greater than 50% of the lumen it is considered thickened
- can be overestimated in Expiratory-phase imaging (due to airway collapse).
- Recognizing segmental anatomy aids in understanding diseases like pneumonia, atelectasis, and bronchiectasis.
Parallels with Human Endeavors
- Segmental bronchi mirror “distribution networks,” such as power grids or water pipelines, where each branch ensures delivery to a specific destination. Like a well-organized system, their failure can disrupt the function of the entire lung.
In a Nutshell
Parts

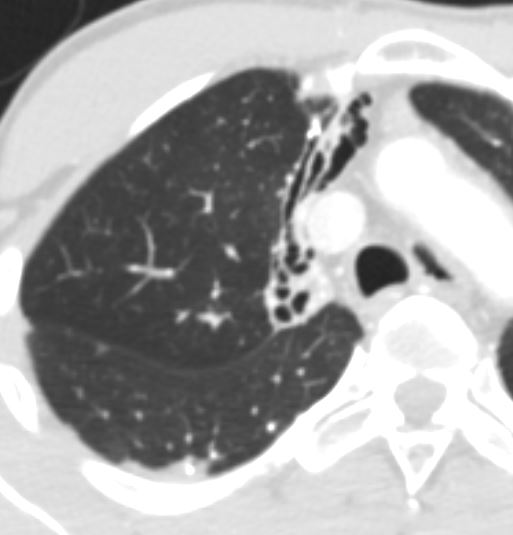
Ashley Davidoff TheCommonvein.net 32686b05L segmental bronchi.8
Size Diameters
There are two lungs and they are made up of lobes which are divided into segments and these are discussed in the individual documents dedicated to each of the lungs.
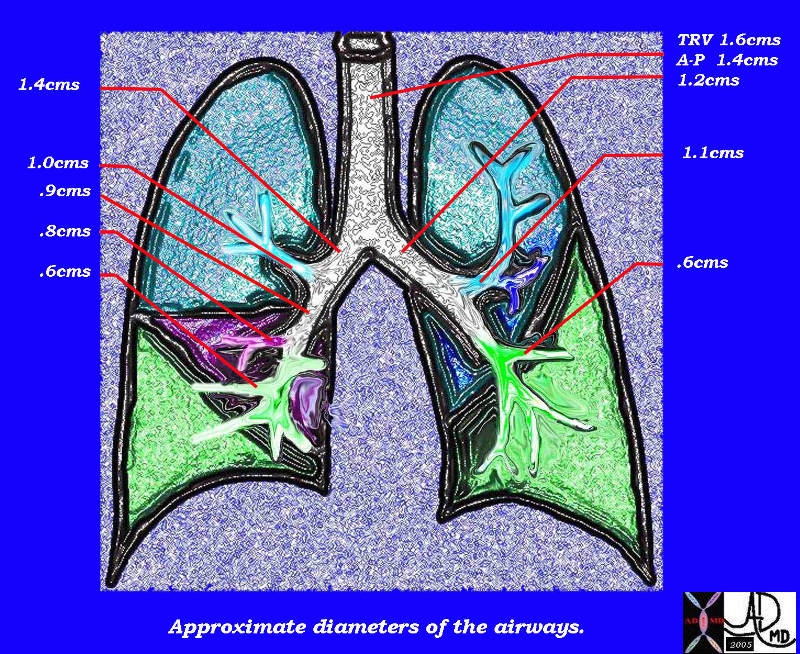
This diagram of the airways reveals the approximate diameters of the airways. Note that the left main stem bronchus is thinner than right whose length is truncated by the take off of the right upper lobe bronchus.
Ashley Davidoff MDTheCommonVein.net
32686b05L05b
Applied Anatomy:
Size
Lobar and Segmental Airway Disease
Ashley Davidoff TheCommonVein.net
Ashley Davidoff TheCommonVein.net
Ashley Davidoff TheCommonVein.net
– Small
Thickening of the Segmental and Subsegmental Airways
Add the 2 walls and if greater than 50% of the lumen = thickened
Bronchitis
33719b.jpg
BronchitisThere is thickening of the segmental and subsegmental airways and mucus in the lumen
Ashley Davidoff TheCommonVein.net
Sarcoidosis
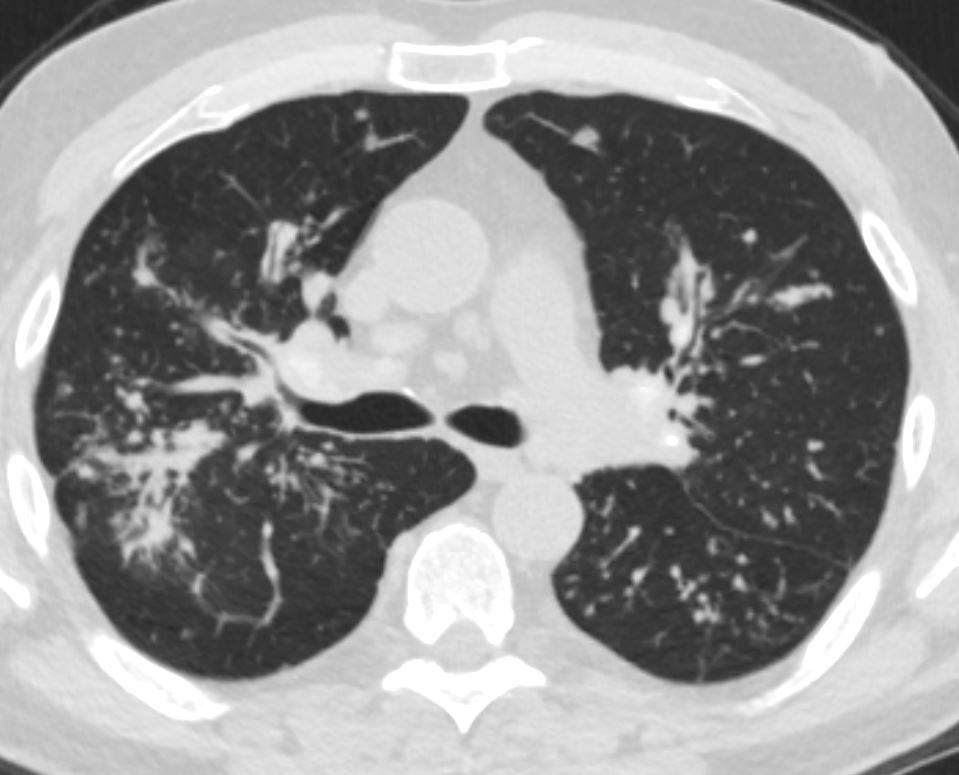
Ashley Davidoff MD The CommonVein.net
sarcoidosis 001 60m
Wegener’s aka Granulomatosis with Polyangiitis
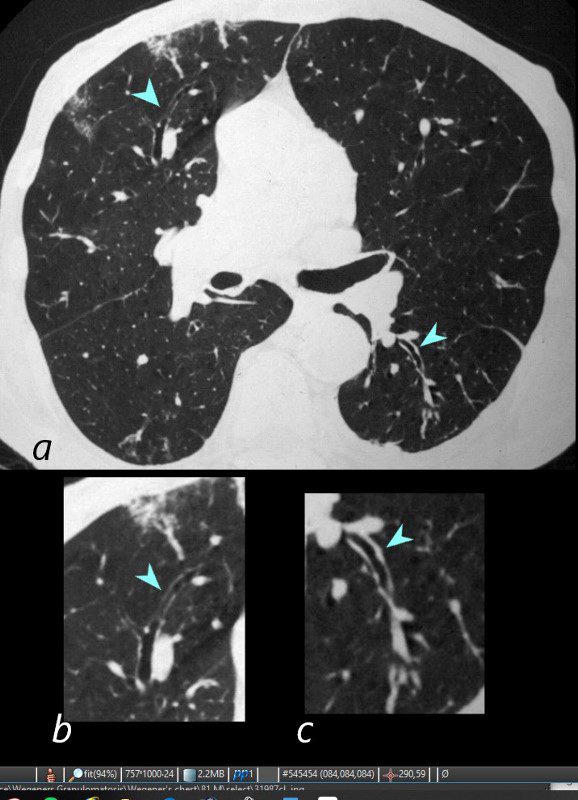
81-year-old male with weight loss, renal failure, and hemoptysis
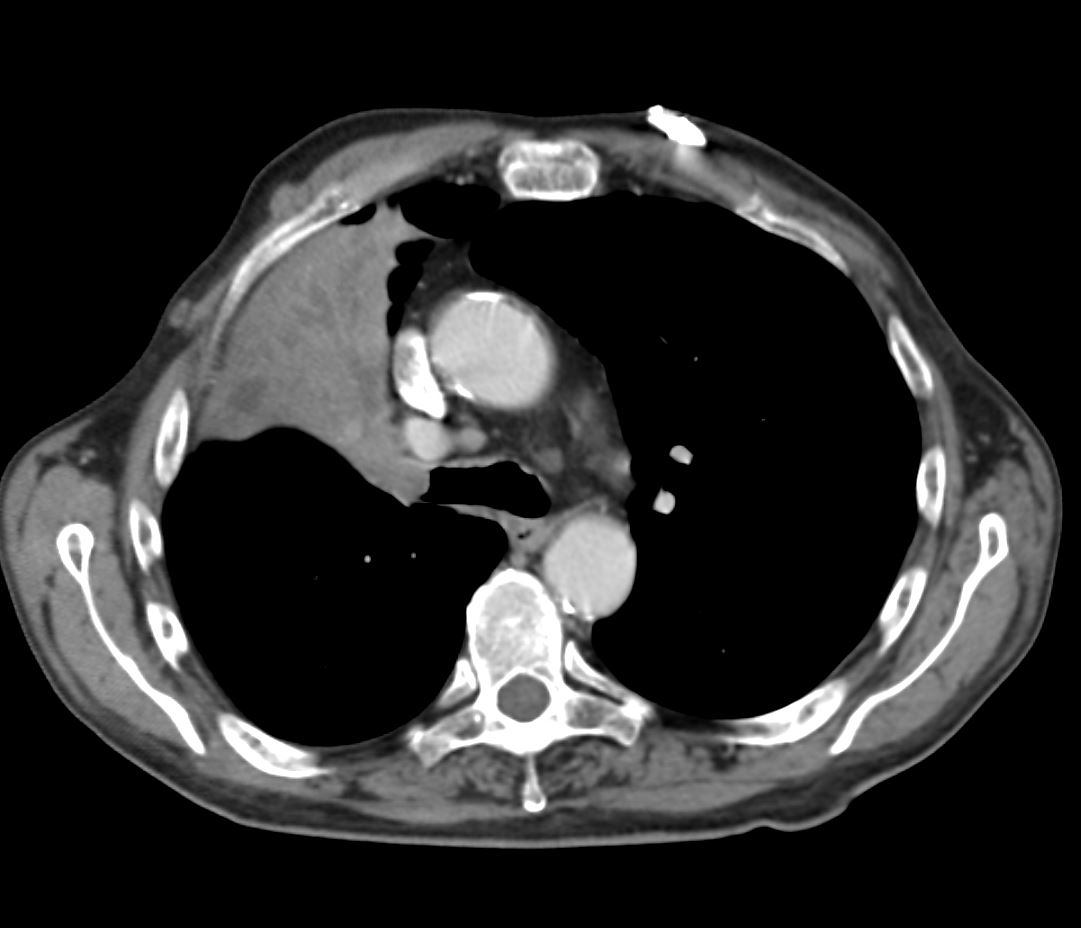
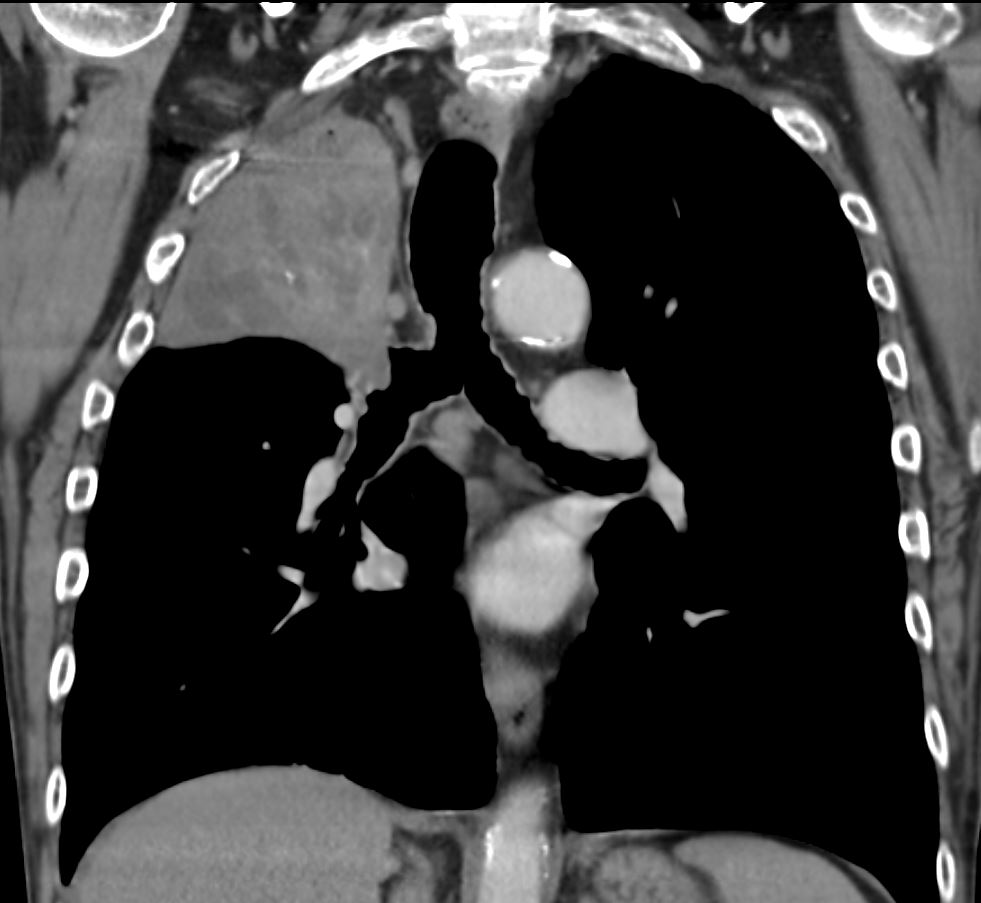
CT axial view (a) shows focal nodular thickening of the segmental and subsegmental airways (a,b,c, teal arrowheads) likely reflecting areas of granulomatous changes.
Priscilla Slanetz MPH MD TheCommonVein.net 31987cLb.8
Applied Anatomy Size – Big
Diameter enlargement of the Segmental Subsegmental and Small Airways
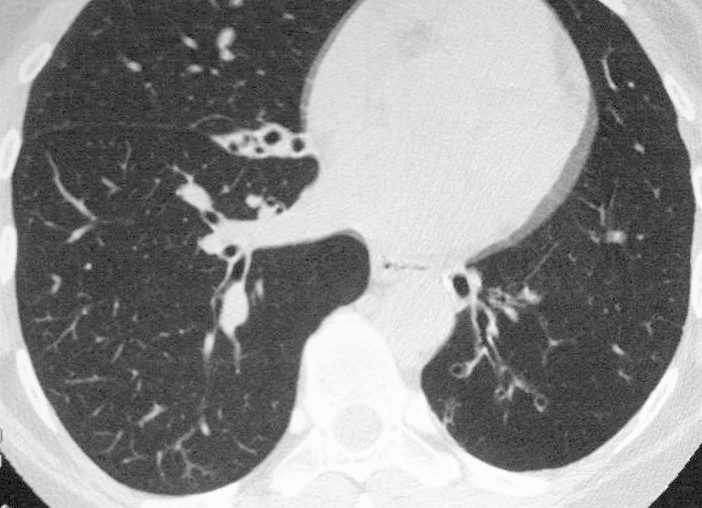
Ashley Davidoff TheCommonVein.net
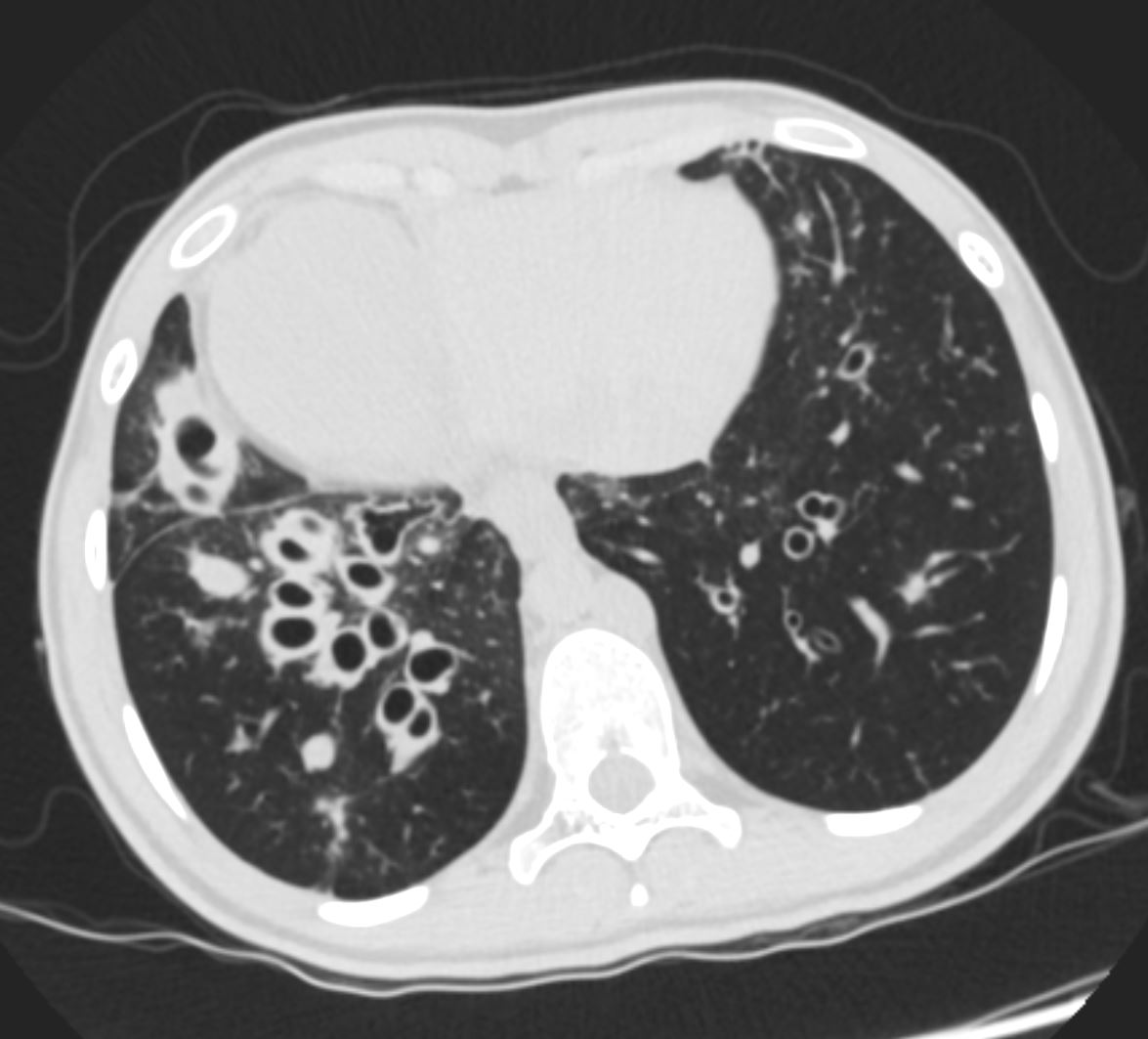
Ashley Davidoff TheCommonVein.net
Applied Anatomy – Traction Bronchiolectasis
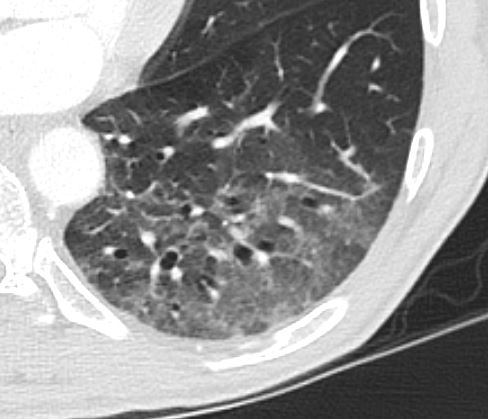
Ashley Davidoff TheCommonVein.net
Position Normal Angles between The Branches
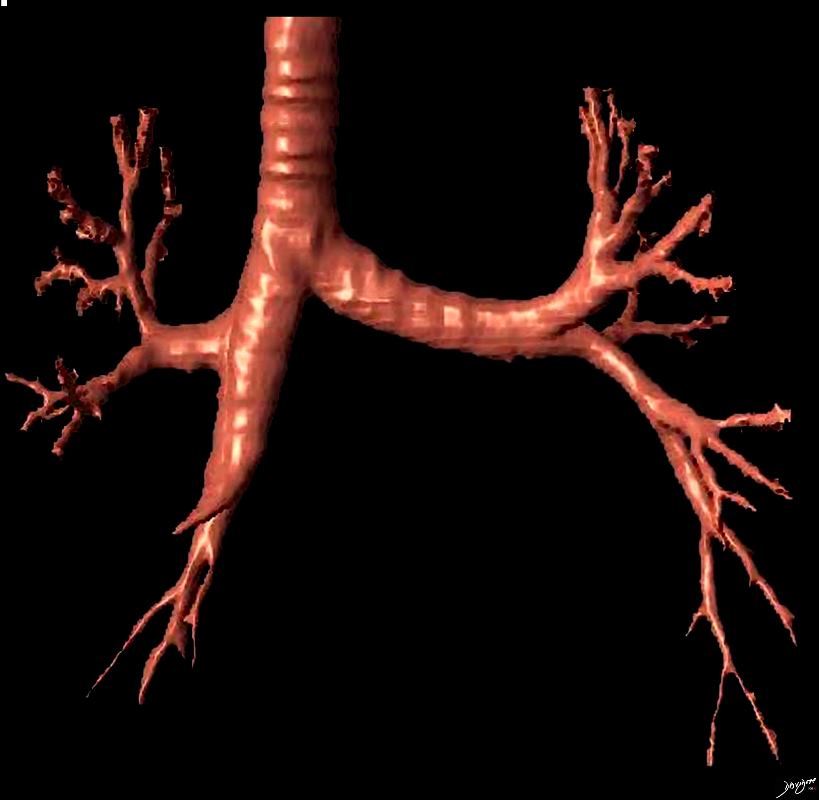
Ashley Davidoff
TheCommonVein.net
Applied Anatomy – Atelectasis
Angles Between the Airways become More Acutes
Character –
Mucoid Impaction
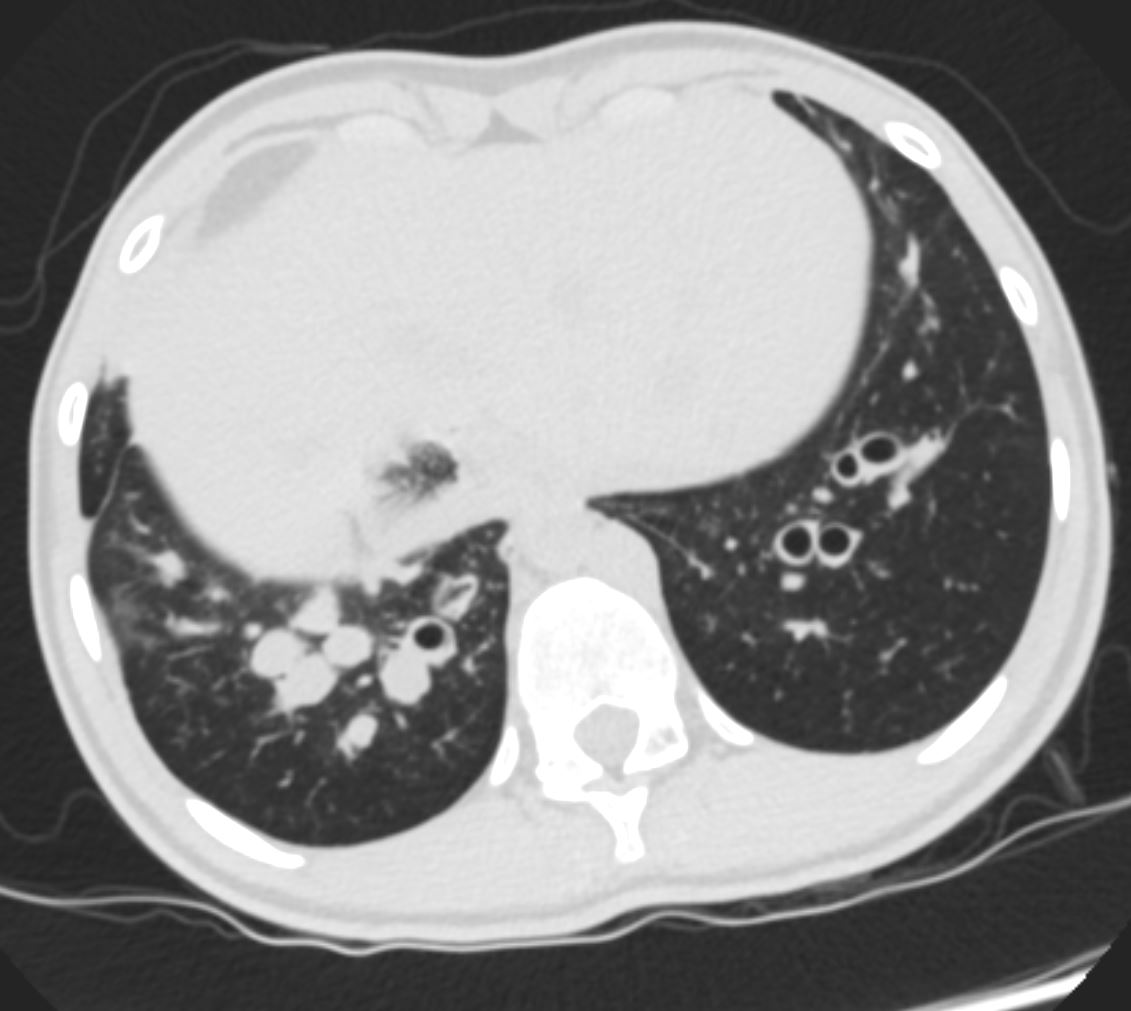
Ashley Davidoff TheCommonVein.net
Bronchocentric Nodules
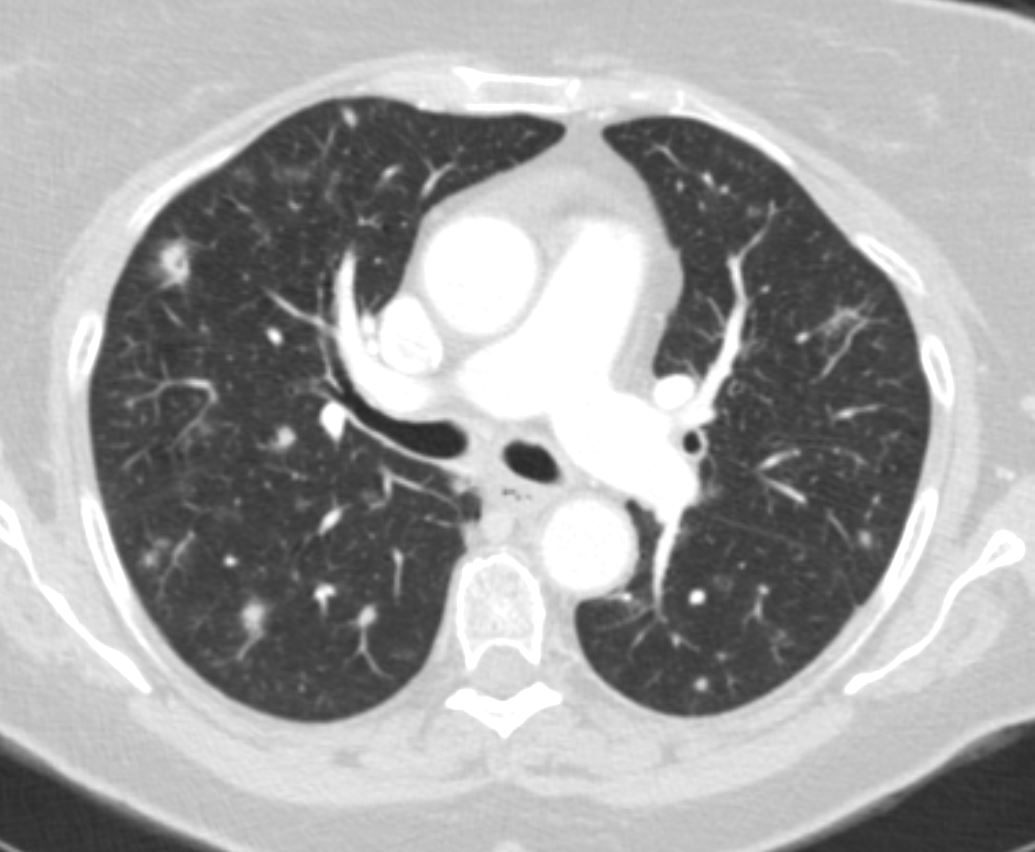
65 F presents 6 years ago with both solid nodules and bronchocentric nodules
Ashley Davidoff MD TheCommonVein.net
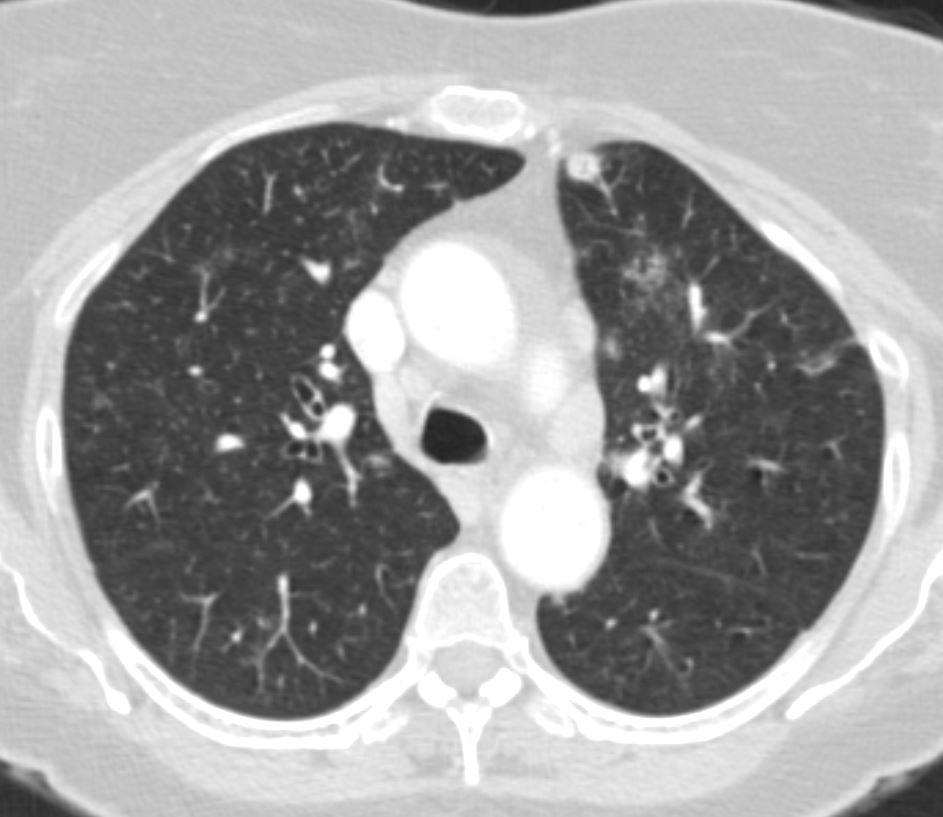
65 F presents 6 years ago with both solid nodules and bronchocentric nodules
Ashley Davidoff MD TheCommonVein.net
Broncholith
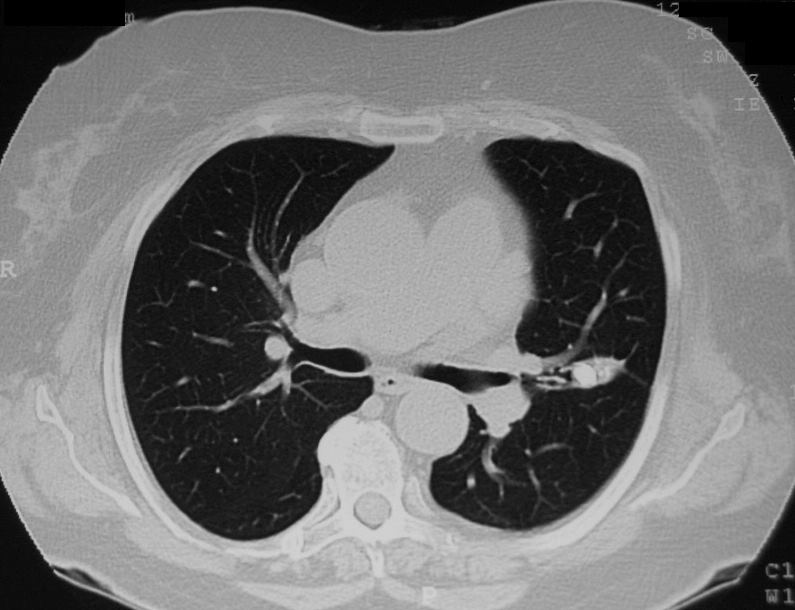
Ashley Davidoff TheCommonVein.net
Time

Courtesy CTisUS
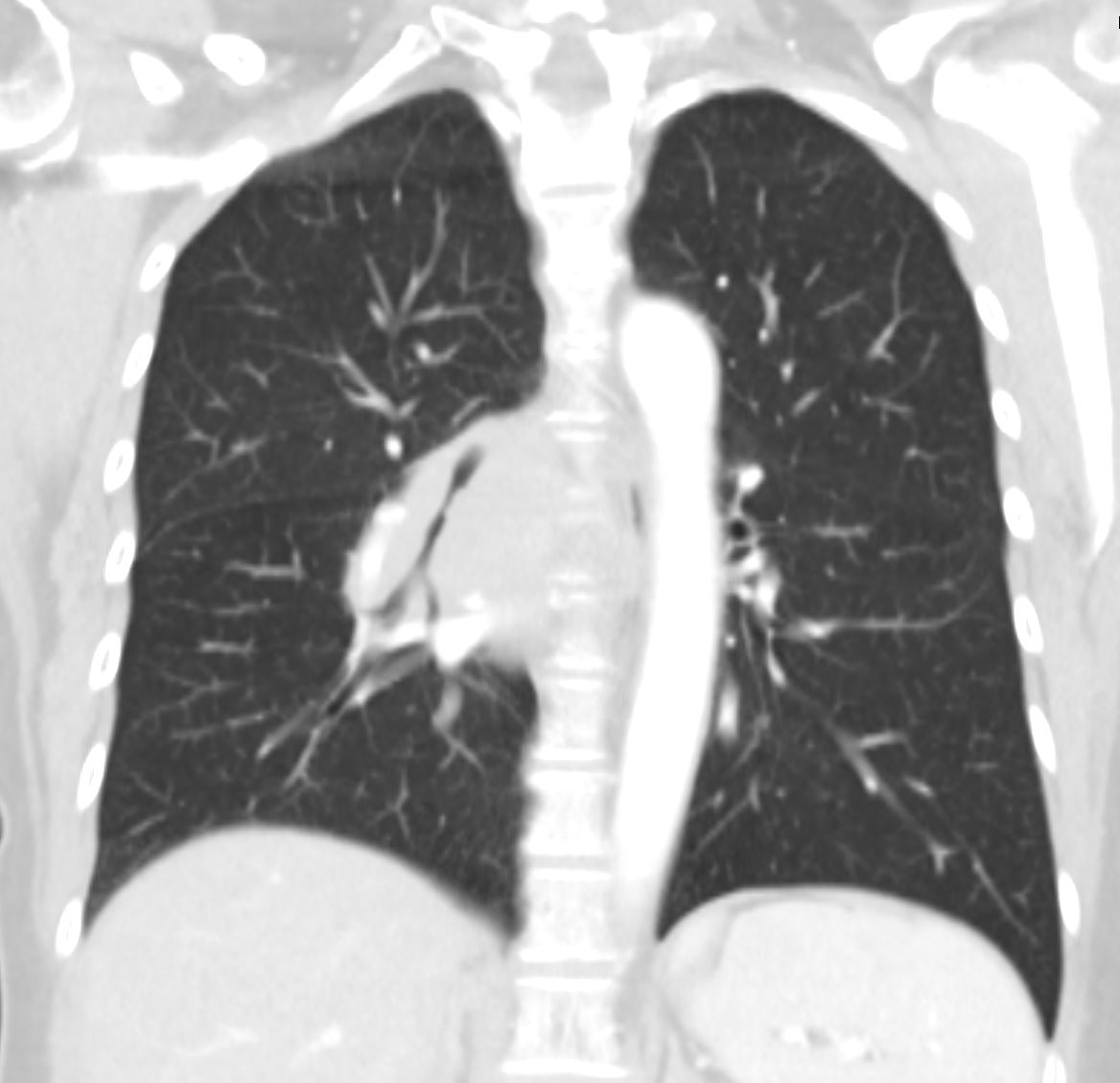
The segments are divided into the secondary lobules. The lobules are made up of the small airways including the terminal bronchioles, respiratory bronchioles, alveolar ducts alveolar sacs and the alveoli themselves.

Grays Anatomy
Right Lung Segments
- Upper lobe
- superior segment
- posterior segment
- anterior segment
- Middle lobe
- lateral segment
- medial segment
- Lower lobe
- apical segment
- medial-basal segment
- anterior-basal segment
- lateral-basal segment
- posterior-basal segment
Left Lung Segments
- Upper lobe
- apico-posterior segment (merger of “apical” and “posterior”)
- anterior segment
- Lingula
- inferior lingular segment
- superior lingular segment
- Lower lobe
- superior segment
- anteromedial basal segment (merger of “anterior basal” and “medial basal”)
- posterior basal segment
- lateral basal segment

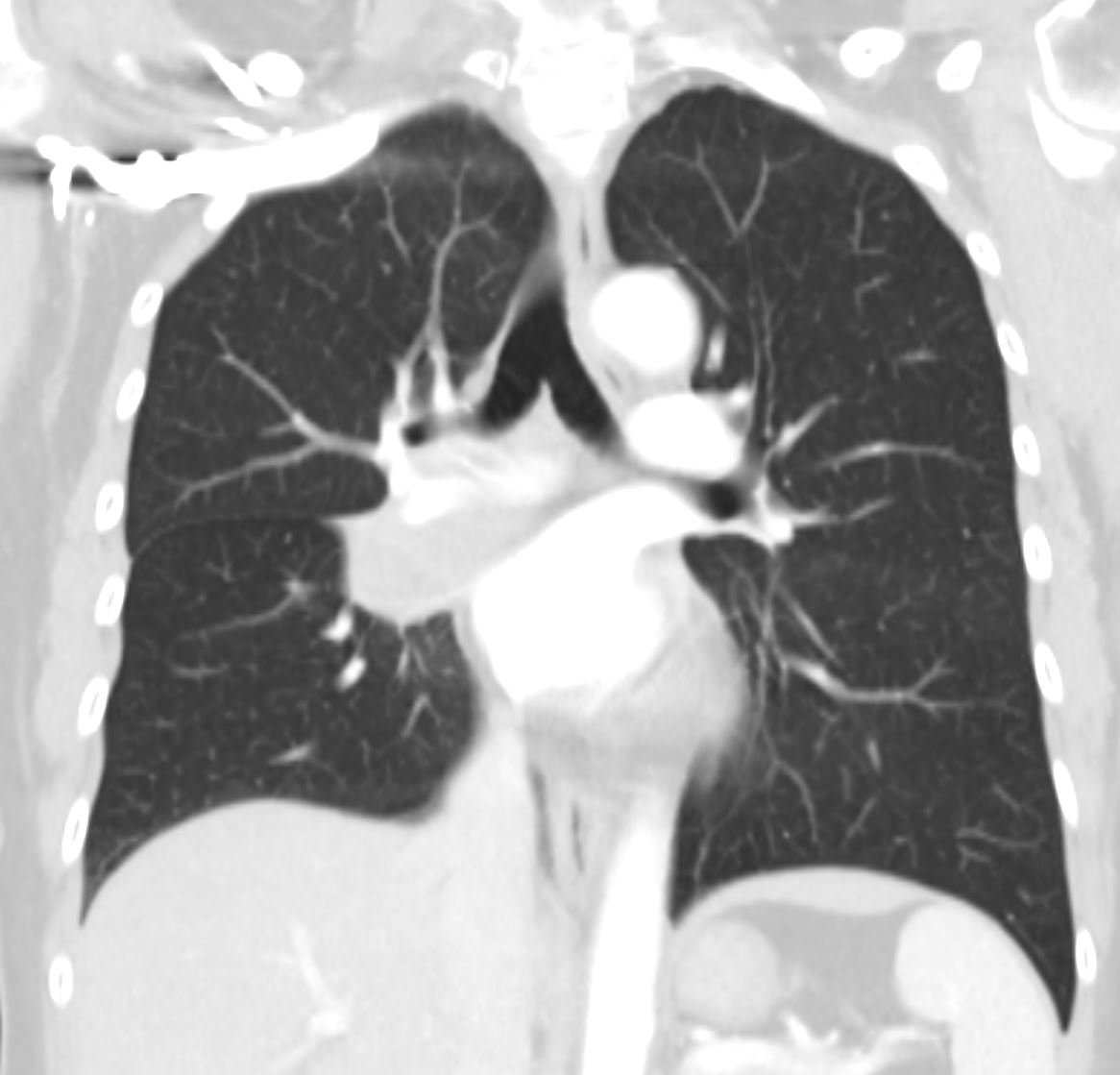
The axial CT through the level of the heart shows a few of the right and left pulmonary segments including parts of the middle lobe, lingula and of the lung bases
Ashley Davidoff MD TheCommonVein.net 42644.800

This diagram of the airways reveals the approximate diameters of the airways. Note that the left main stem bronchus is thinner than right whose length is truncated by the take off of the right upper lobe bronchus.
Ashley Davidoff MDTheCommonVein.net
32686b05L05b
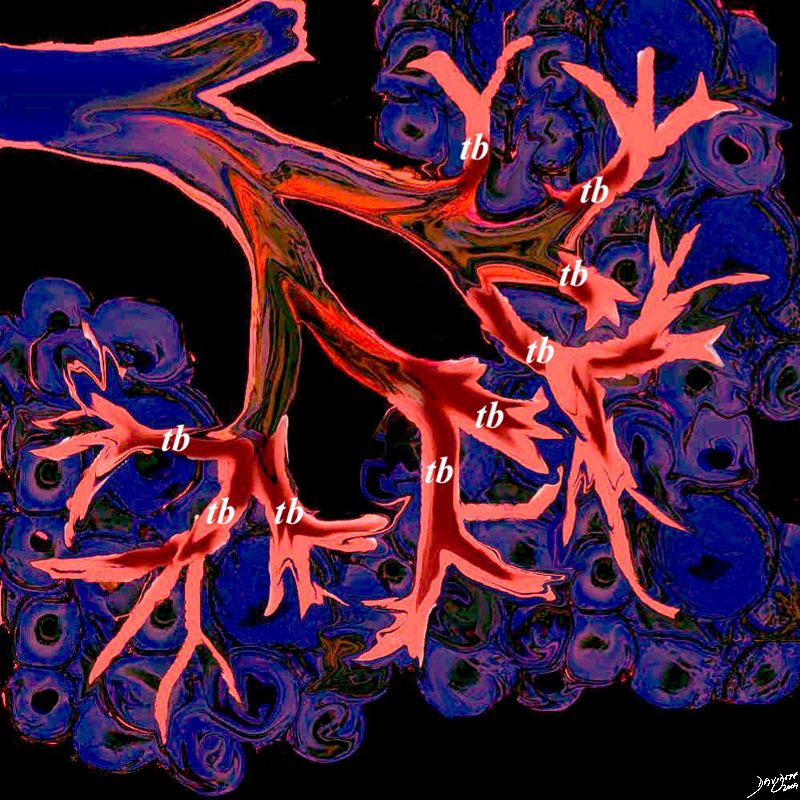
This artistic rendition of the small parts of the lung shows the beginning of the peripheral system just before it enters the acinus. This duct is called the terminal duct and it is the last part of the ductal system that has no ability for gas exchange. After its first division, the bronchioles become the respiratory bronchioles, and they are the first in the system to have an ability to both transport the gases as well as enable gas exchange.
Ashley Davidoff TheCommonVein.net 32645b04b05.8s

Airways are lined by a pseudostratified ciliated columnar epithelium interspersed with mucus secreting goblet cells
Ashley Davidoff
TheCommonVein.net lungs-00674b01-lo res
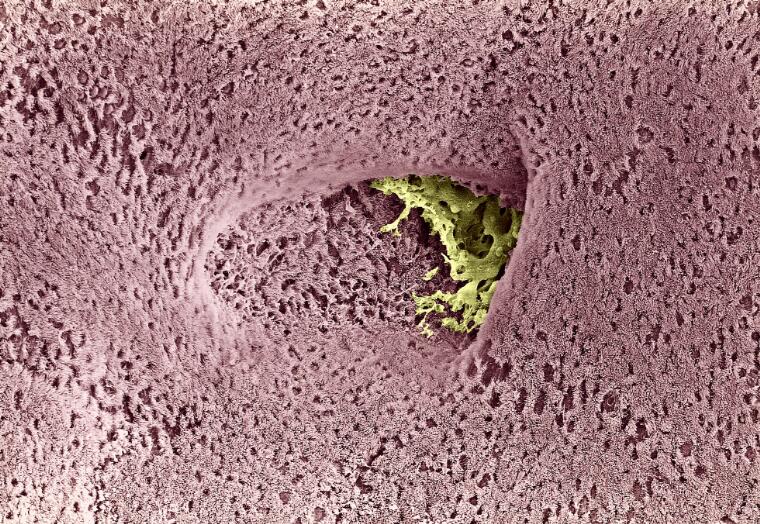
David Gregory and Debbie Marshall
Licence: Attribution 4.0 International (CC BY 4.0)
Credit: Bronchus of the lung.
David Gregory and Debbie Marshall.

Ashley Davidoff
TheCommonVein.net
lungs-00675-lo-res
David Gregory & Debbie Marshall
Licence: Attribution 4.0 International (CC BY 4.0)
RUL bronchus is about 1 cm in diameter, with the apical segment being 4-7mm, and the RML bronchus is about 8mms. The LUL bronchus resembles its uncle, the right main stem bronchus, since it is also relatively short and fat possibly because it is responsible to give rise to the lingula as well as the left apical segments. It measures 11mm mm in diameter (but only 9mm in length). The ascending upper division bronchus is approximately 7 mm in diameter.
General diameters of the downstream airways include lobular and segmental bronchi (5-8mm), subsegmental bronchi and bronchiole (1.5-3mm), lobular bronchiole (1mm), terminal bronchiole (.7mm) and acinar bronchiole (.5mm). (Webb Muller Naidich)The acinus is about 7-15 mms in diameter.
From the lobular bronchus there are 9 to 14
dichotomous branches. Lobular bronchus has 3 to 5 smaller
airways, which are called terminal bronchioles
“A unit consisting of 3 to 5 terminal bronchioles supplied by a
small bronchus with a diameter of 1 mm is called a secondary
pulmonary lobule (Reid?s lobule) (Reid 1958)”.
Applied Anatomy
In Part 1 we learned how radius and flow were related. When the flow is laminar as it is in the smaller airways Poiseuille?s law applies which states the following
Volume flow rate = pressure difference
resistance
= P1-P2
R
= pressure difference X radius4
8/pi viscosity X length
Thus it can be seen that a small change in the radius makes a major difference in the volume flow rate. Number of divisions and length are other key considerations in flow but they do not carry the same power as radius. Narrowing of the airways at any level significantly affects delivery of air downstream.
The failure of air delivery is one of the major causes of fatality during an anaphylactic reaction or a severe asthma attack. Return of airway patency is the first consideration in resuscitation. In fact, in any resuscitation, the “A” of the “ABC” of resuscitation is to ensure patency of the airway first.
Maintenance of airway diameter is provided by cartilage in the upper components of the airways. In the trachea a C-shaped cartilaginous ring surrounds the anterior and lateral walls while the posterior wall consists of a membrane allowing for some pliability during the phases of respiration. The trachea lengthens and dilates during inspiration while it shortens and narrows during expiration.
Some disease states compromise the patency of the airways. Tracheomalacia is a softening of the cartilage of the trachea and during inspiration the trachea will be unable to maintain its diameter. This will result in a relative collapse during inspiration and hence diminished air flow.
More downstream, support of airway patency transforms from cartilagenous support to muscular support. Asthma, inflammation, or infection can affect the diameter by inducing muscle spasm while the presence of edema of the wall would also result in narrowing of the lumen. In most of these diseases the airways of both lungs are diffusely involved and respiratory decompensation can easily result. Collapse of a lobe, segment, or sub segment of a lung on the other hand may not affect respiratory function at all when the remaining lung is normal or near normal. Localized collapse may occur with foreign body inhalation, mucus or purulent impaction, and tumor growth. Patients who have undergone a pneumonectomy normal pulmonary function usually exists, unless there is disease in the other lung.
Segmental and (Often) Subsegmental Disease
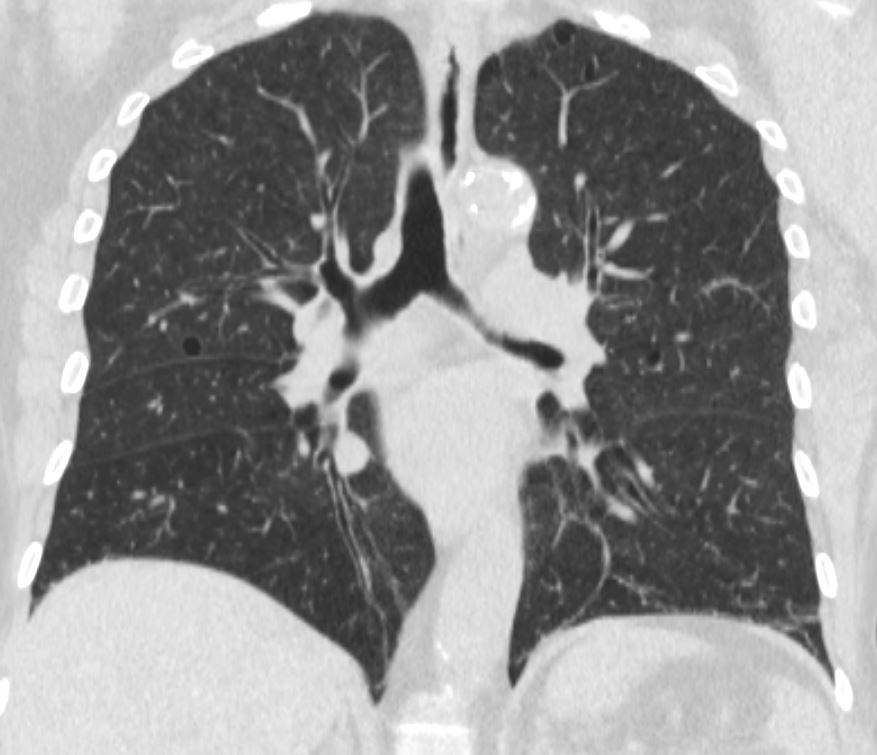
Ashley Davidoff MD TheCommonVein.net airways-68F

70217c01 lung bronchus bronchi left mainstem bronchus lower lobe bronchi thickened walls strand of mucus dx chronic bronchitis character mucus CTscan Davidoff MD

81-year-old male with weight loss, renal failure, and hemoptysis
CT axial view (a) shows focal nodular thickening of thesegmental and subsegmental airways (a,b,c, teal arrowheads) likely reflecting areas of granulomatous changes.
Priscilla Slanetz MPH MD TheCommonVein.net 31987cLb.8
Segmental and Subsegmental Disease
Extending into the Small Airways

lung axial interstitium bronchioles connective tissue fx bronchial plugging peribronchial halo peribronchial thickening dx bronchopneumonia CTscan
Ashley Davidoff MD. The Common Vein.net 47614c01

47114c01 bronchi lungs fx dilated enlarged impacted with sft tissue finger in glove dx allergic broncho-pulomonary aspergillosis ABPA aspergillus dx infection inflammation CTscan Davidoff MD
Bronchiectasis
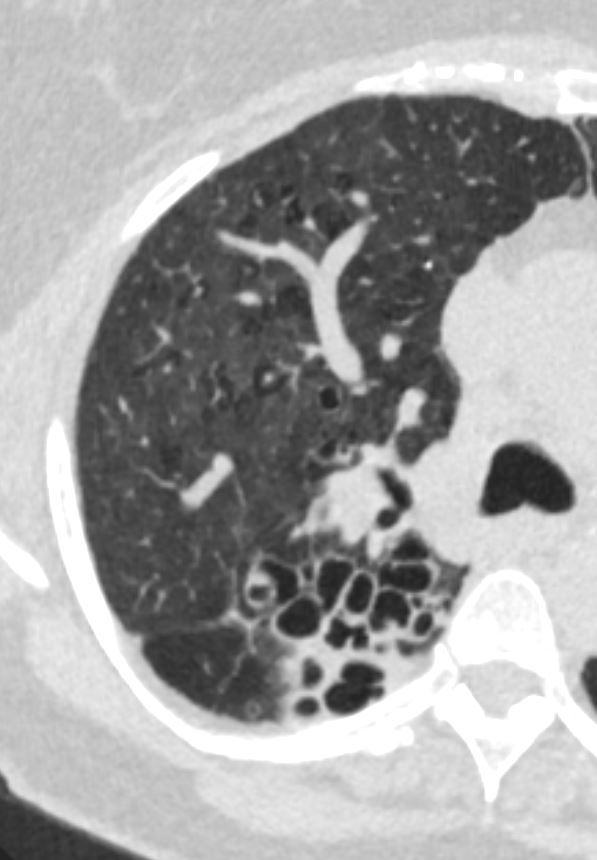
Ashley Davidoff MD TheCommonVein.net bronchiectasis 006b

Ashley Davidoff MD TheCommonVein.net bronchiectasis 005
In the normal patient the pulmonary arterial branches and the bronchi are about the same size until they reach the hallowed halls of the pulmonary lobule.

In this normal CXR a RUL segmental bronchus and artery are side by side with the lucent air filled bronchus in teal and the artery in royal blue. Note that at his stage they are the same size and they will be for many divisions until they reach the terminal bronchiole. Courtesy Ashley Davidoff MD 42464b07
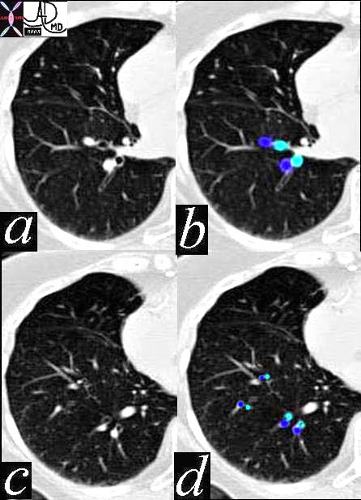
In this CT of a normal patient we see two levels of the RLL with the segmental bronchioles and arterioles branching dichotomously and simultaneously. Note again the similarity in size and shape through these levels of division, a form that is maintained in the normal person until they reach the terminal bronchiole.
Ashley Davidoff MD TheCommonVein.net 42455
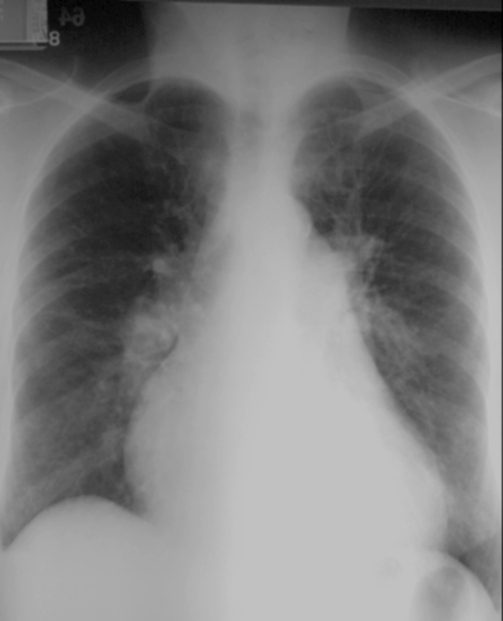
This is a plain CXR of a patient in congestive heart failure. There are a few diagnostic findings that confirm the diagnosis including the large heart the distribution of the vessels to the upper lobes and the indistinct vascular borders. What do you think of the size of the right upper lobe segmental artery that is seen in cross section?
Ashley Davidoff MD. TheCommonVein.net
15439
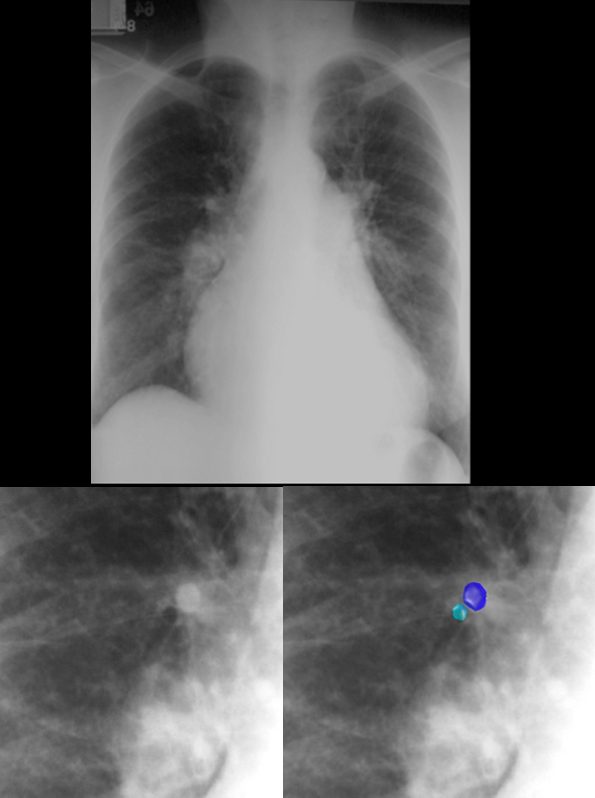
This patient is in heart failure with cephalization of the vessels, increase in the artery to bronchus ratio in the upper lobe bronchovascular bundle, (lower image – teal = bronchus and royal blue = artery) and perhaps mild early interstitial edema characterized by fuzziness of the right lower pulmonary artery.
The heart is enlarged and the pulmonary artery is enlarged characterizing pulmonary hypertension. Ashley Davidoff MD TheCommonVein.net
The reason we are able to see the air filled bronchi within the air filled lung is because they have wall that is made up of the soft tissue allowing for an interface. As the bronchi get smaller their walls will get proportionately thinner until they are too thin to resolve at which time they will blend into the parenchyma. They become invisible as discrete structures on high resolution CT when they reach 2mm in size, which corresponds to the bronchioles that are about 2cms from the lung periphery. The arterioles on the other hand maintain their soft tissue character and their interface with air of the lung is maintained allowing detection even in subpleural regions of the lung periphery.
Bronchiectasis is a condition where disease within the walls of the airways results in weakening resulting in enlargement and hence ectasia. In this condition therefore the bronchioles will be larger than their arteriole counterpart and they will be seen within 1cms distance of the pleura.

This patient is in heart failure with cephalization of the vessels, increase in the artery to bronchus ratio in the upper lobe bronchovascular bundle, (lower image – teal = bronchus and royal blue = artery) and perhaps mild early interstitial edema characterized by fuzziness of the right lower pulmonary artery.
The heart is enlarged and the pulmonary artery is enlarged characterizing pulmonary hypertension.
Ashley Davidoff MD TheCommonVein.net 15439c.8
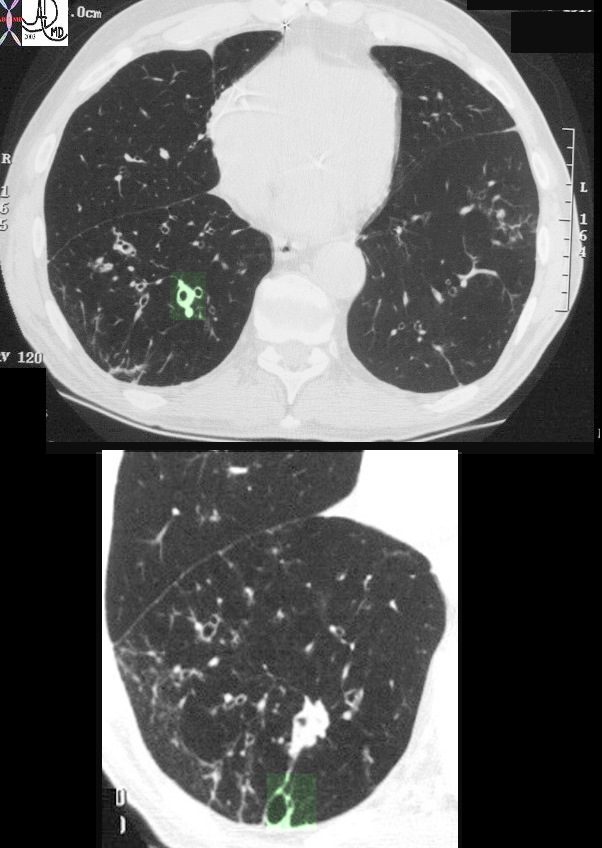
This image represents the CT scan of the chest with a focus on the right lower lobe in an 86year old patient who presented with long standing history of a productive cough. The segmental airways in the upper image are thickened (green overlay) as are the subsegmental airways in the lower panel. Additionally an small airways is abnormally visualised within 1cms of the pleura, indicating traction bronchiolectasis. Aspiration is a radiological consideration
Ashley Davidoff MD TheCommonVein.net 30602c
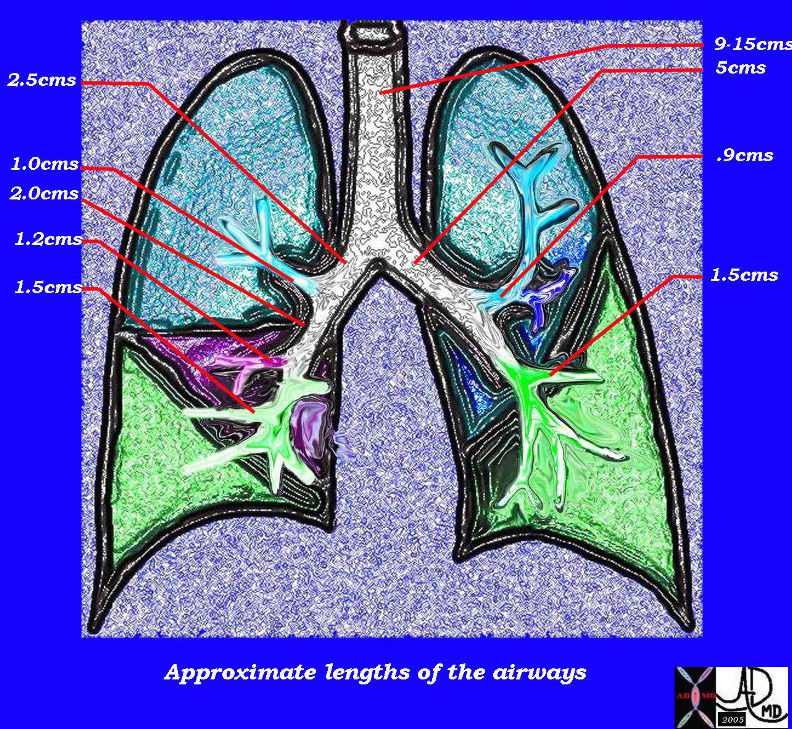
This diagram of the airways reveals the approximate lengths of the airways. Note that the left main stem bronchus is about twice the length of the right whose length is truncated by the take off of the right upper lobe bronchus.
Courtesy Ashley Davidoff MD
TheCommonVein.net
32686b05L04b
Other Important Dimensions in the Airways
Number of Divisions
The right lung has 10 bronchopulmonary segments while the left has 8. There are approximately 23 airway divisions from the mainstem bronchi to the level of the alveoli.
The number of branches from hilum to periphery is variable with the shortest path to a terminal bronchiole being about 7 divisions and total length of 7 to 8 cms, and the longest pathway having about 25 branch divisions with total length of more than 22 cm. In the upstream portions of the airways the tree does not divide as frequently as they do downstream. The lungs are large organs and the aim of the airways is to deliver the air to the alveoli. Distance needs to be covered from the hilum where alveoli are relatively sparse, to the periphery where the alveoli are abundant. Prior to entering the secondary lobule the bronchovascular bundle branches at approximately 1cm intervals. Once they enter the confines of the lobule branching becomes fast furious and a new branch originates every 1-3mms. This frequency of division in the hallowed halls of gas exchange makes intuitive sense as surface area becomes the dominant focus.
Thickness
The walls of the bronchi change in character and in thickness as the airways progress downstream. The following are the measured thicknesses of the downstream airways;
| lobular and segmental bronchi | 1.5mm |
| subsegmental bronchi and bronchiole | .2-.3mm |
| lobular bronchiole | .15mm |
| terminal bronchiole | .1mm |
| acinar bronchiole | .05mm |
Area
Cross-sectional area of the airways
Trachea approximately 2.5 cm2
Alveoli approximately 11,800 cm2
Time
Gas exchange takes 0.25 seconds or 1/3 of the total transit time of a red cell under resting conditions. The entire blood volume of the body passes through the lungs each minute in the resting state, that is 5 liters per minute.
Applied Anatomy
Knowledge of the relationship of the trachea and esophagus is extremely important for those who are intubating patients or placing nasogastric tubes. The trachea and esophagus lie very close to each other, and so it is not uncommon for the intubations to be misplaced.
Upper lobe disease such as TB will cause fibrosis and shrinkage of the lung structures with consequent traction effect and elevation of the hilum so that it is pulled upward.
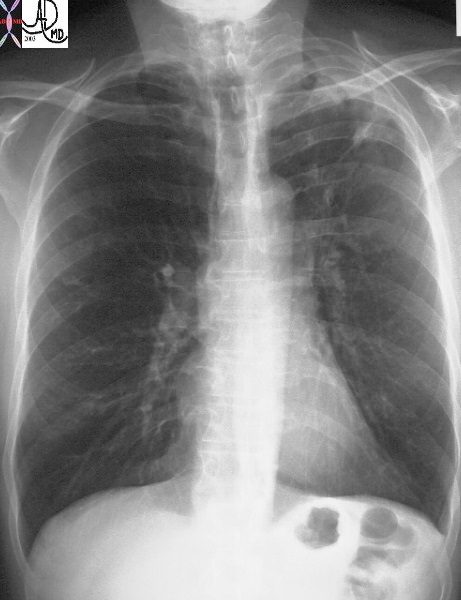
The CXR of this patient shows retraction of the left hilum so that it lies higher than it should. Linear atelectasis identified in the LUL. This correlates with the finding on the CT accounting for the volume loss and elevation of the left hilum. Ashley Davidoff MD TheCommonVein.net 30562
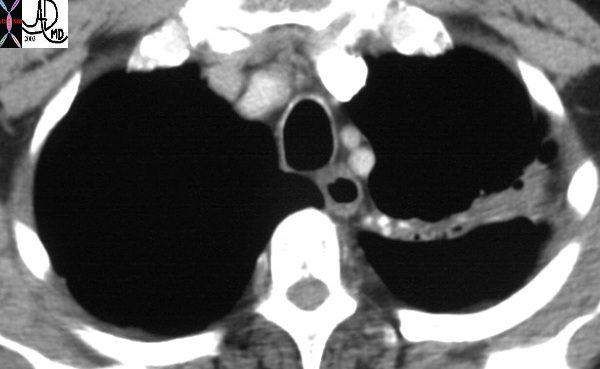
In this patient with TB there is a linear band like density with calcifications in the LUL characteristic of atelectatic change in the RUL. This loss of volume is associated with fibrosis and retraction seen on the CXR in the following image. Ashley Davidoff MD TheCommonVein.net 30557
Lymphangitis Carcinomatosis
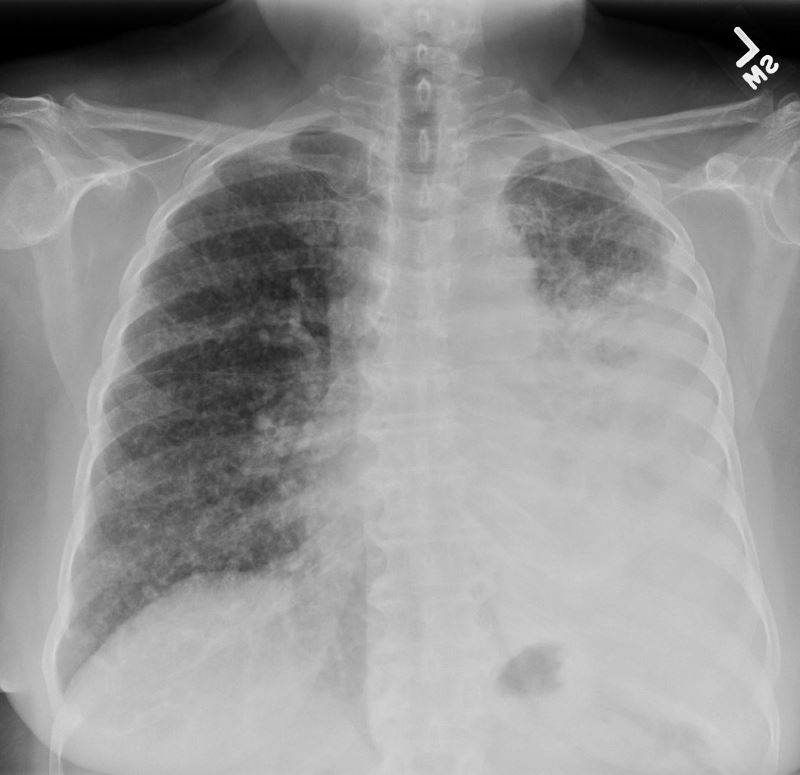
50 year old female with primary adenocarcinoma of the left lung with diffuse bilateral lymphangitic spread of disease characterized by lymhovascular distribution.
The nodularity on the fissures characterize the lymphatic distribution and the nodules are likely of a mixed nature, some being in the iinterlobular septa, and some in a centrilobular distribution Ashley Davidoff MD TheCommonVein.net
131028.jpg
ADENOCARCINOMA OF LEFT LUNG WITH BILATERAL LYMPHANGITIC SPREAD50 year old female with primary adenocarcinoma of the left lung with diffuse bilateral lymphangitic spread of disease characterized by lymphovascular distribution.
The nodularity on the fissures characterize the lymphatic distribution and the nodules are likely of a mixed nature, some being in the interlobular septa, and some in a centrilobular distribution .
Ashley Davidoff MD TheCommonVein.net
Elevated Right Main Stem Bronchus and Encasement

Ashley Davidoff MD The CommonVein.net
sarcoidosis 001 60m
Amyloid Involvement

Ashley Davidoff
TheCommonVein.net 44f Amyloid airways 002
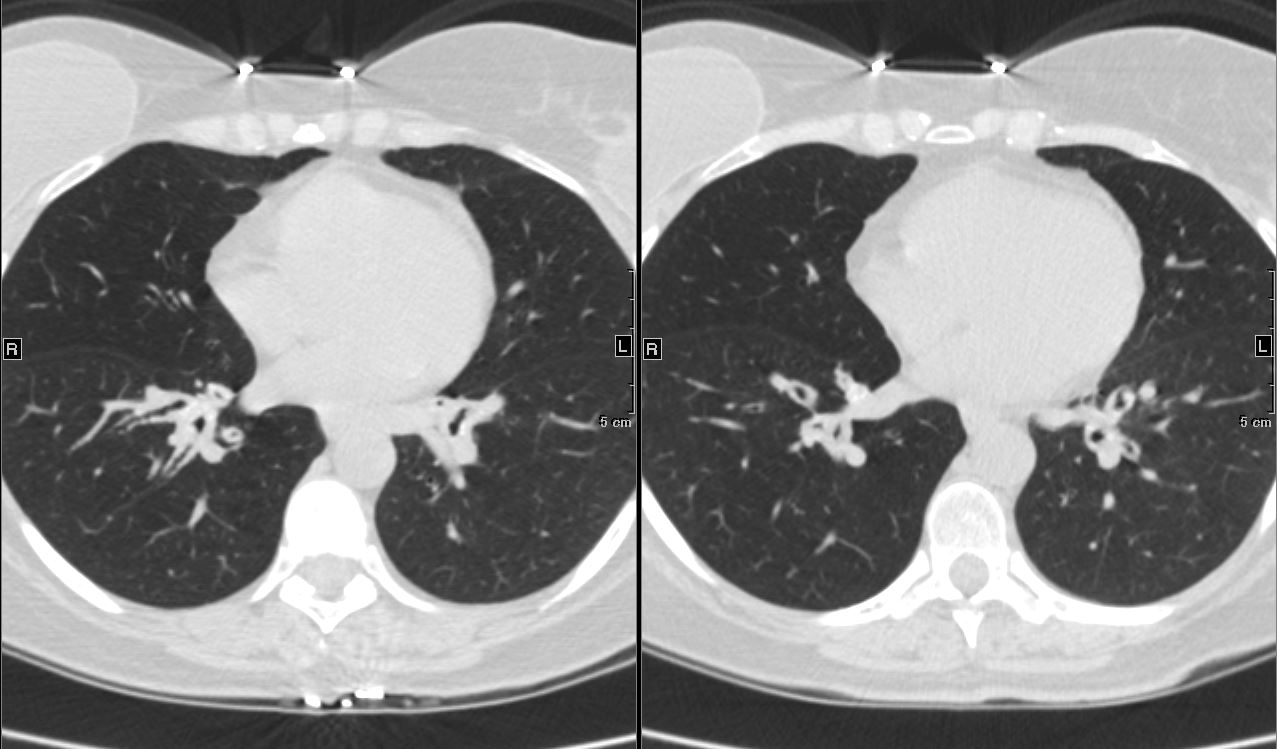
Ashley Davidoff
TheCommonVein.net 44f Amyloid airways 005

Axial CT image shows involvement of known amyloid in the segmental airways Ashley Davidoff MD
TheCommonVein.net amyloid-airways-002