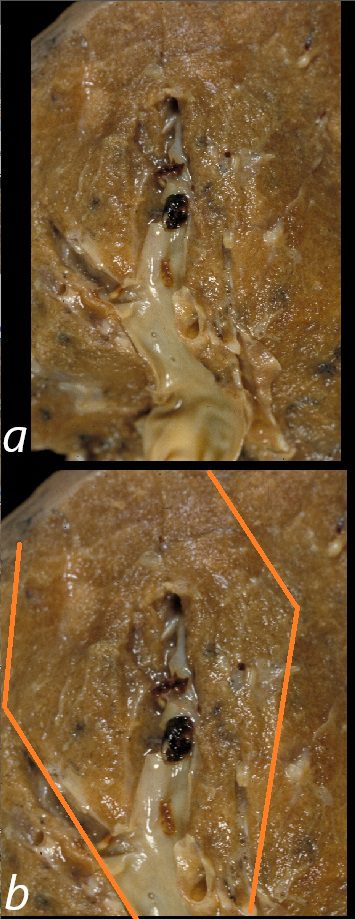
This is a post mortem specimen of a lung in a patient who had primary lung carcinoma with metastatic liver disease, portal vein thrombosis, a small pulmonary embolus and a pulmonary infarct in the LUL. . There is a subtle discoloration of the wedge shaped region (outlined in b ) and the adjacent lung reflecting the distribution of the ischemic/infarcted region. The radiology correlate on an X-ray or CT scan would be called a Hampton’s hump 32191cL
PE pulmonary infarction
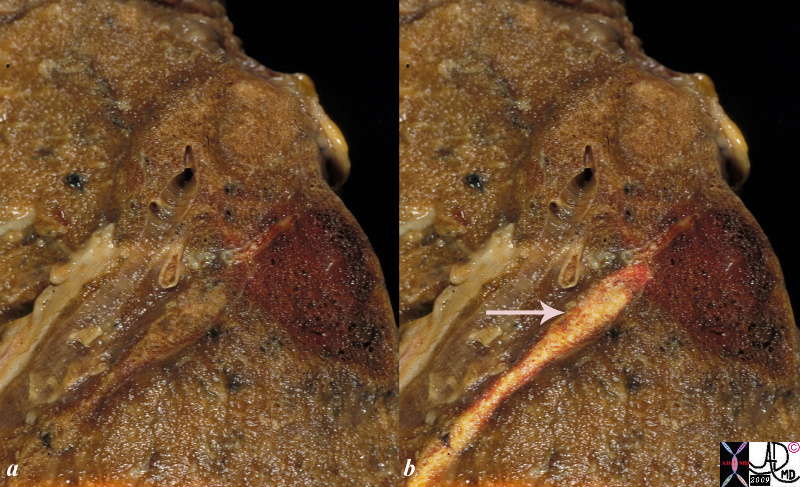
This is a post mortem specimen of a lung in a patient who had primary lung carcinoma with metastatic liver disease, portal vein thrombosis, a pulmonary embolus and a pulmonary infarct This autopsy specimen shows a wedge shaped hemorrhagic infarction alongside a similarly sized swollen wedge shaped subsegment of lung. The artery subtending the hemorrhagic portion contains a cream colored thrombus (highlighted in b – arrow) and suggests a subacute thrombus The radiology correlate on an X-ray or CT of these side by side wedge shaped lesions would be called a “Hampton’s hump”
Pathophysiology
Thrombus Formation: Most commonly originates in the deep veins of the lower extremities.
Embolization: The thrombus dislodges and travels through the venous system to the right heart and pulmonary arteries.
Vascular Obstruction:
Reduces blood flow in the pulmonary circulation.
Increases pulmonary artery pressure and right ventricular workload.
Ventilation-Perfusion (V/Q) Mismatch:
Ventilated areas of the lung are not perfused, leading to hypoxemia.
Circulatory
Main Pulmonary Arteries
Saddle Embolus
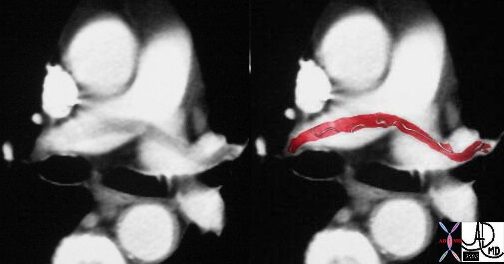
This case of a saddle embolus shows a thrombus sitting astride the left and right pulmonary arteries. Contemporary CTA is able to identify emboli in secondary and tertiary branches just as well. CTA has become the gold standard and study of choice in the patient with chest pain or acute desaturation with suspected PE.
Ashley Davidoff MD TheCommonVein.net 30008c

CT in the axial plane in a patient with acute dyspnea and chest pain shows embolic filling defects almost occluding the right pulmonary artery and partially occluding the left pulmonary artery consistent with acute occluding pulmonary emboli
Ashley Davidoff MD TheCommonVein.net 86257c
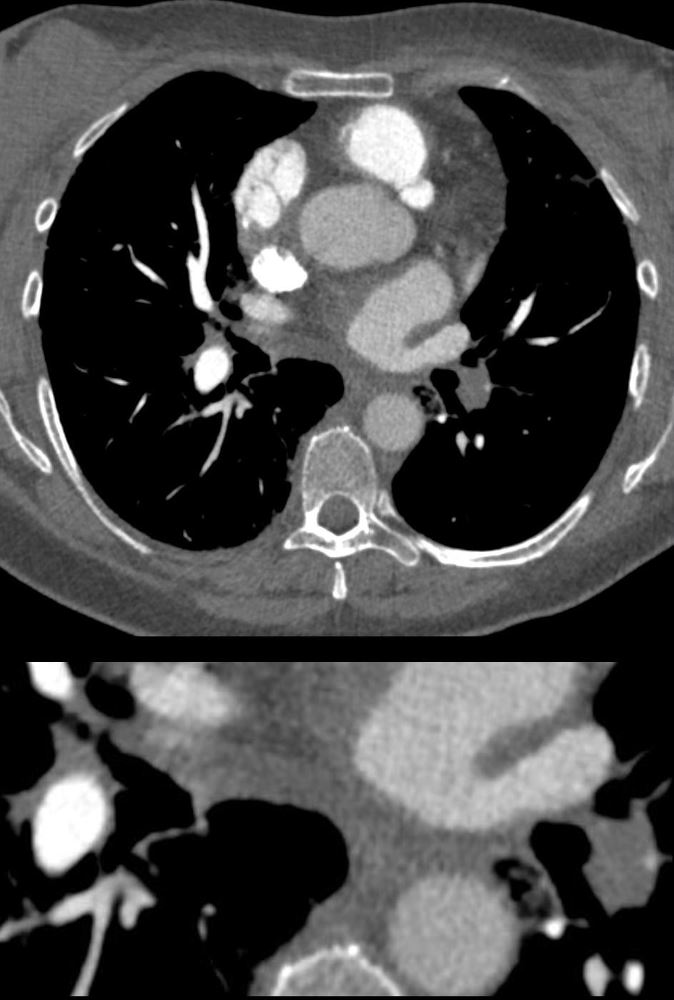
56 -year-old female with a history of amyloidosis presenting with tachycardia and dyspnea. CTPA shows an occlusive embolus (PE) in the left lower lobe pulmonary artery.
Ashley Davidoff MD TheCommonVein.net 135738c
Mismatched Ventilation- Perfusion (V/Q) Scan Multiple Bilateral Pulmonary Emboli

28-year-old female on OCP with leg swelling, chest pain and dyspnea.
Previously performed CXR was normal. Perfusion scan (above) shows multiple bilateral perfusion defects which are not matched on the ventilation scan (below). These findings are consistent with multiple pulmonary emboli
Ashley Davidoff MD TheCommonVein.net 274Lu 11006c02

28-year-old female on OCP with leg swelling, chest pain and dyspnea.
Previously performed CXR was normal. Perfusion scan (a) shows multiple bilateral perfusion defects (white arrowheads) which are not matched on the normal ventilation scan (b). These findings are consistent with multiple pulmonary emboli. CT scan through the upper and mid portions of the chest (c,d) confirm the presence of multiple occlusive and non-occlusive pulmonary emboli magnified and ringed (e,f)
Ashley Davidoff MD TheCommonVein.net 274Lu 11006c03L
PE and No Enhancement of the Left Lower Lobe Arterial Segments and Small Wedge Shape Infarction (Hamptons Hump)
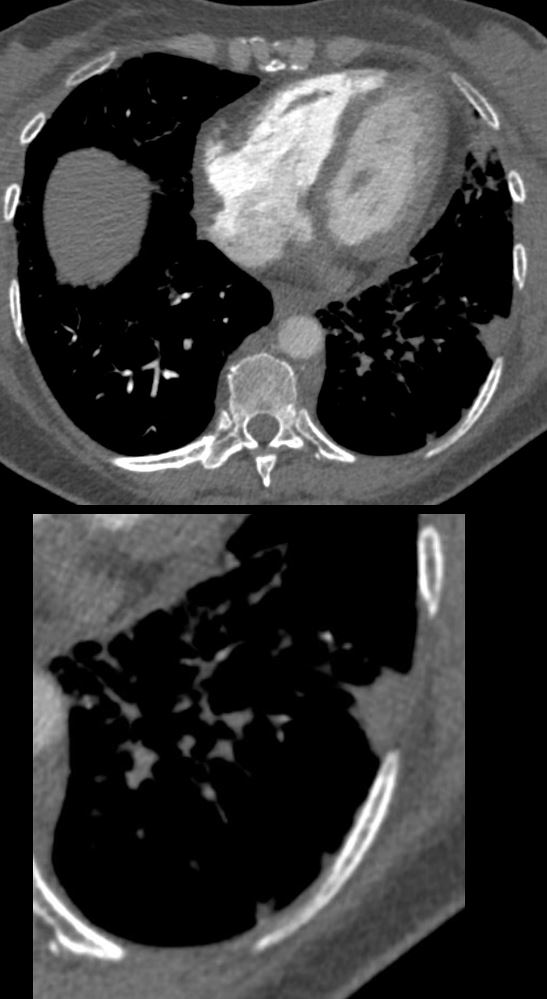
56 -year-old female with a history of amyloidosis presenting with tachycardia and dyspnea. CTPA shows no contrast enhancement of the pulmonary arteries subtending the left lower lobe compared to the right and a subsegmental wedge shaped defect (Hampton?s hump) in the lateral segment of the left lower lobe
Ashley Davidoff MD TheCommonVein.net 135739c
PE and No Enhancement of the Left Lower Lobe-
Dual Energy Iodine Map
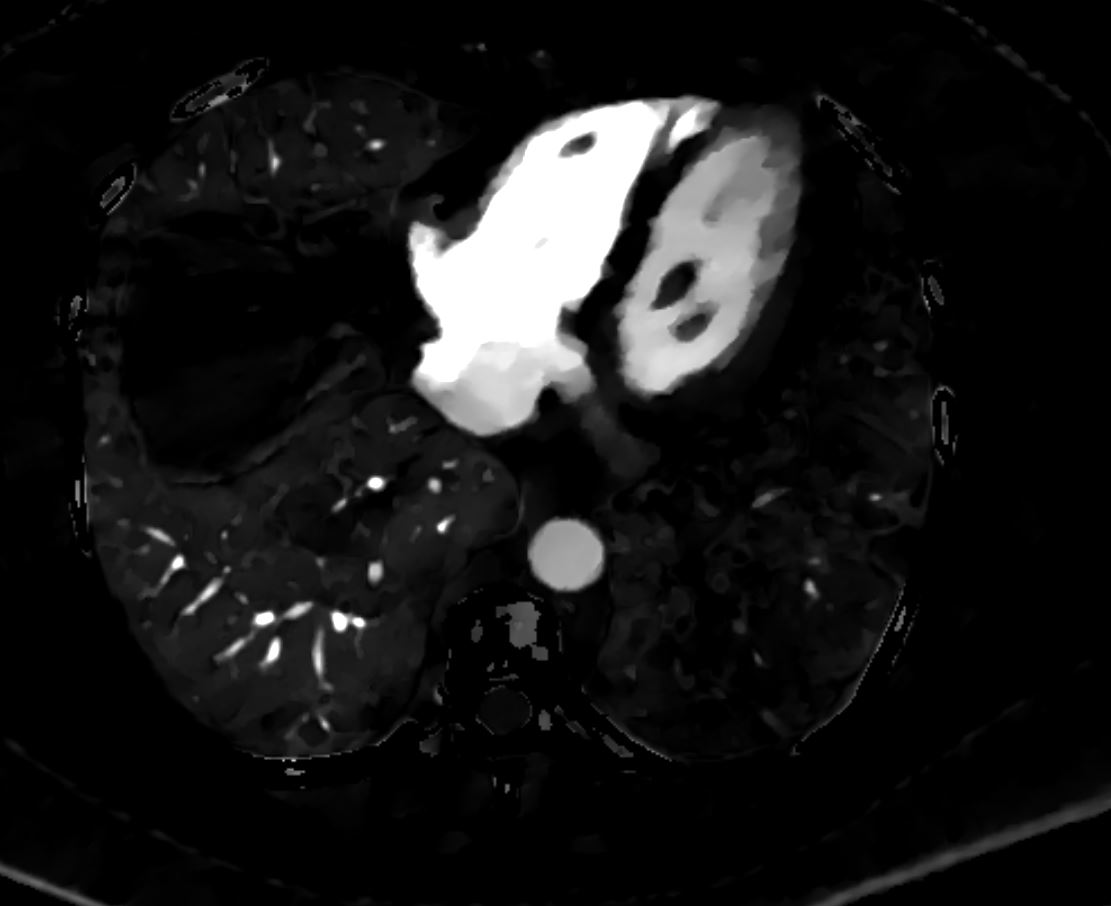
Perfusion Defect of the Left Lower Lobe from Occlusive Pulmonary Embolus
56 -year-old female with a history of amyloidosis presenting with tachycardia and dyspnea. Dual energy CT with an iodine map shows shows an almost lobar perfusion defect of the left lower lobe compared
Ashley Davidoff MD TheCommonVein.net 135740
Subsegmental Infarction
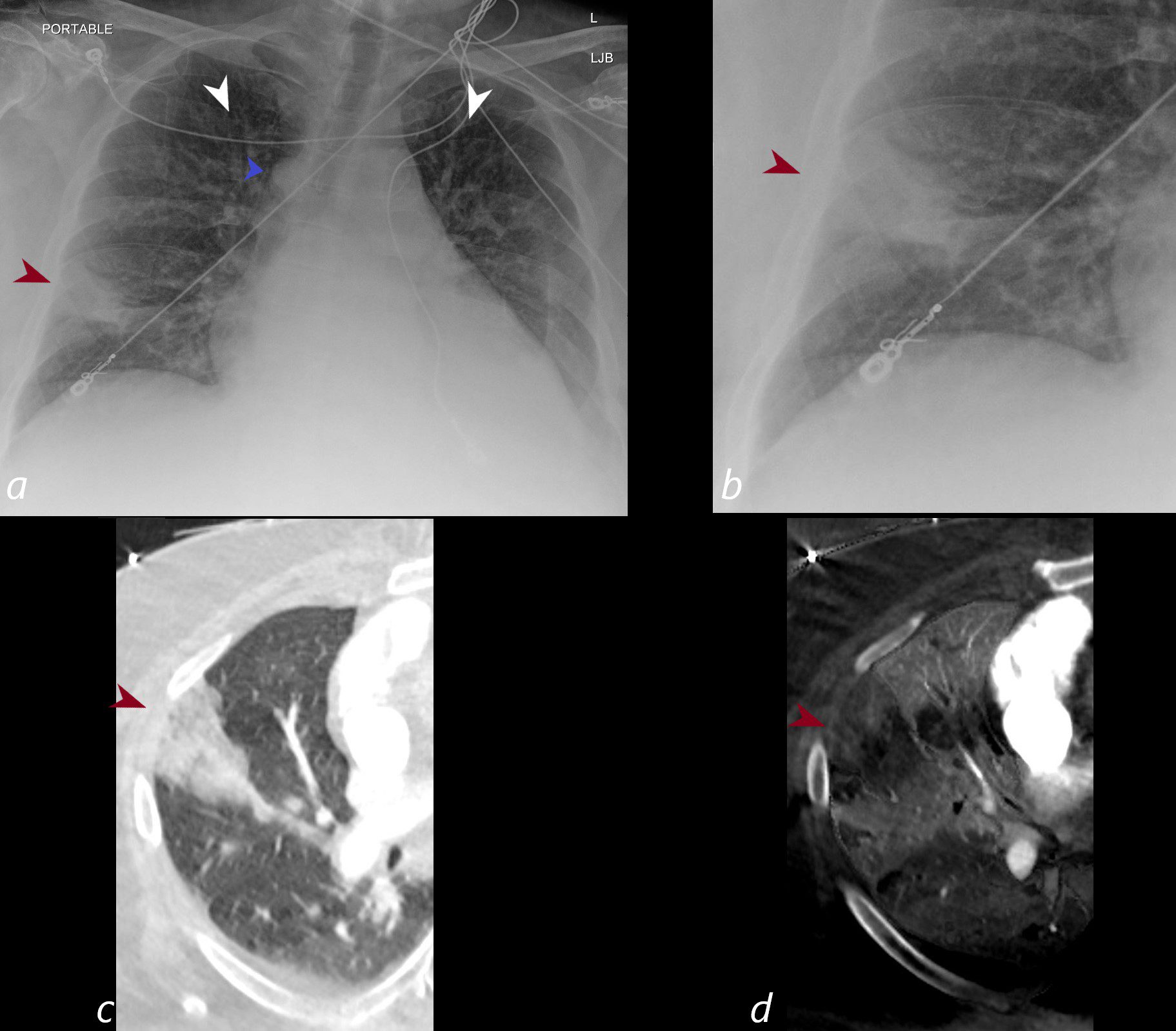
CXR shows a wedge shaped infiltrate in the middle lobe of the lung secondary to a pulmonary embolus (PE) characteristic of a Hampton’s hump (maroon arrowheads a,b) The infarction is confirmed on the CT with contrast (maroon arrowhead c) as well as the region of a perfusion defect (d- maroon arrowhead) In addition there is evidence of CHF on the CXR with cephalization of the vessels (white arrowheads c) cardiomegaly with left atrial enlargement, and enlargement of the azygous vein (blue arrowhead a)
Ashley Davidoff MD TheCommonVein.net)
Segmental Infarction

Patient presented with dyspnea and chest pain. CTPA shows large pulmonary embolus subtending a region of right lower lobe infarction.
Ashley Davidoff MD TheCommonVein.net 19443L
Septic Emboli
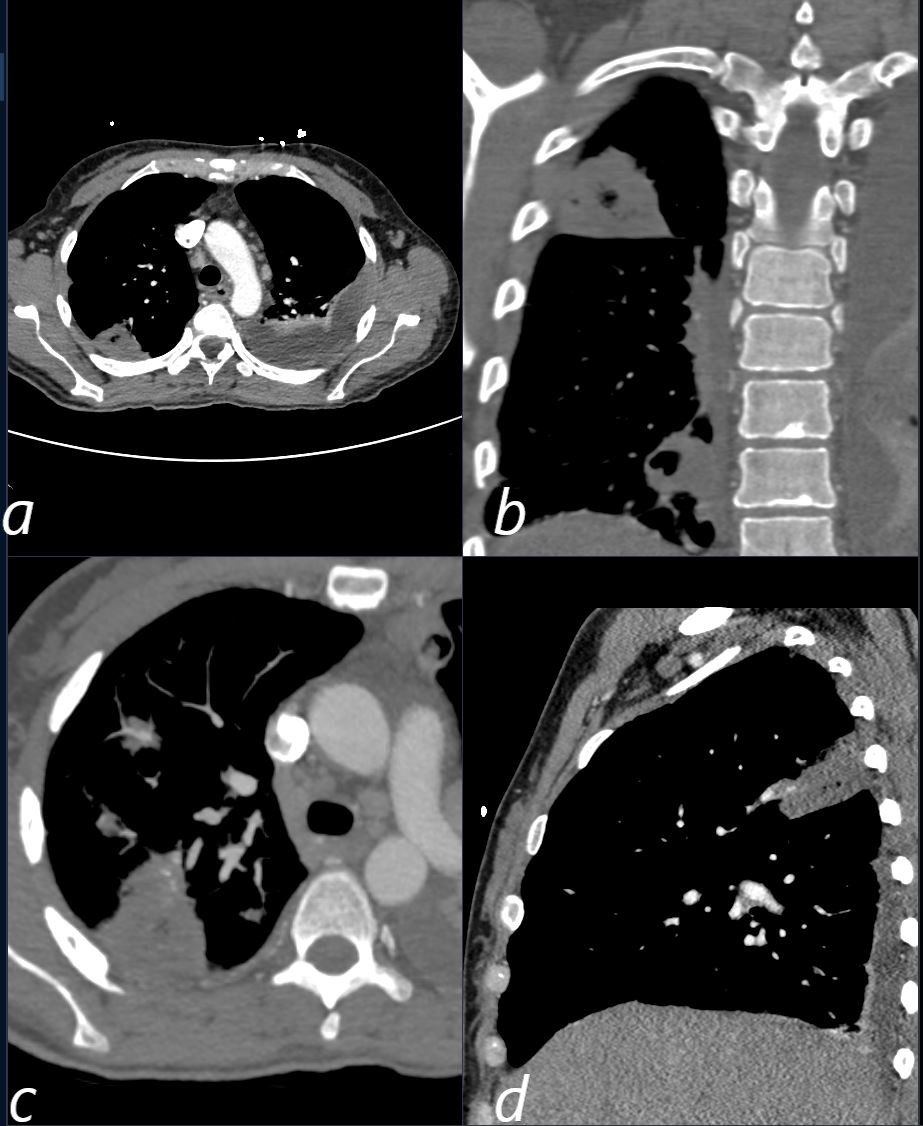
CT scan in a 39 year old female with endocarditis presents with a fever and right sided chest pain.
Multiple views in axial (a,c) coronal (b) and sagittal reveals the presence of a wedge shaped consolidation with cavitation confirming the presence of an infected and cavitating infarction in the posterior segment of the left upper lobe. A loculated effusion is noted at the left base.
Ashley Davidoff TheCommonVein.net
b11422c
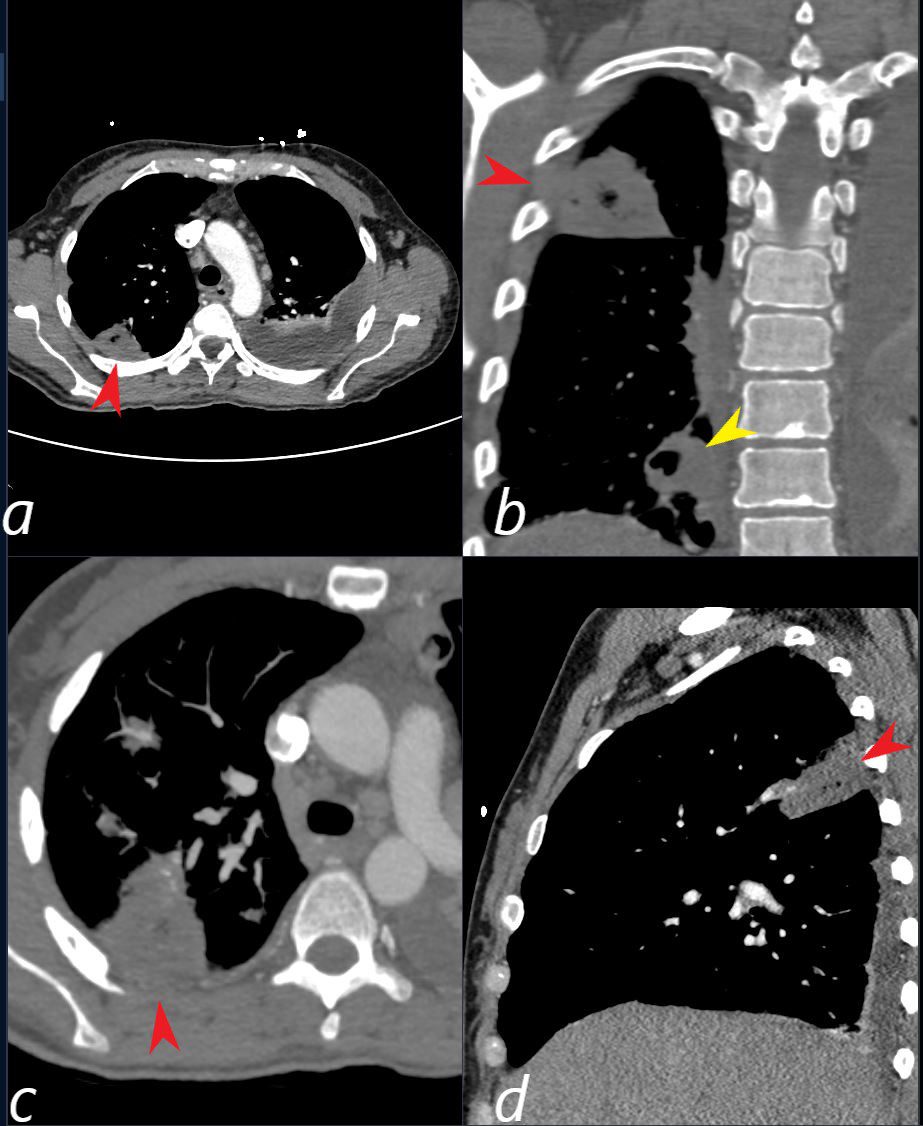
CT scan in a 39 year old female with endocarditis presents with a fever and right sided chest pain.
Multiple view in axial (a,c) coronal (b) and sagittal confirm the presence of a wedge shaped consolidation with cavitation (red arrowhead a,b,c, and d) confirming the presence of an infected and cavitating infarction in the posterior segment of the left upper lobe. A second similar subsegmental infarct and abscess (yellow arrowhead, is noted in the right lower lobe (b yellow arrowhead) . A loculated effusion is noted at the left base.
Ashley Davidoff TheCommonVein.net b11422cL
Unusual Septic Emboli With Cystic Necrosis
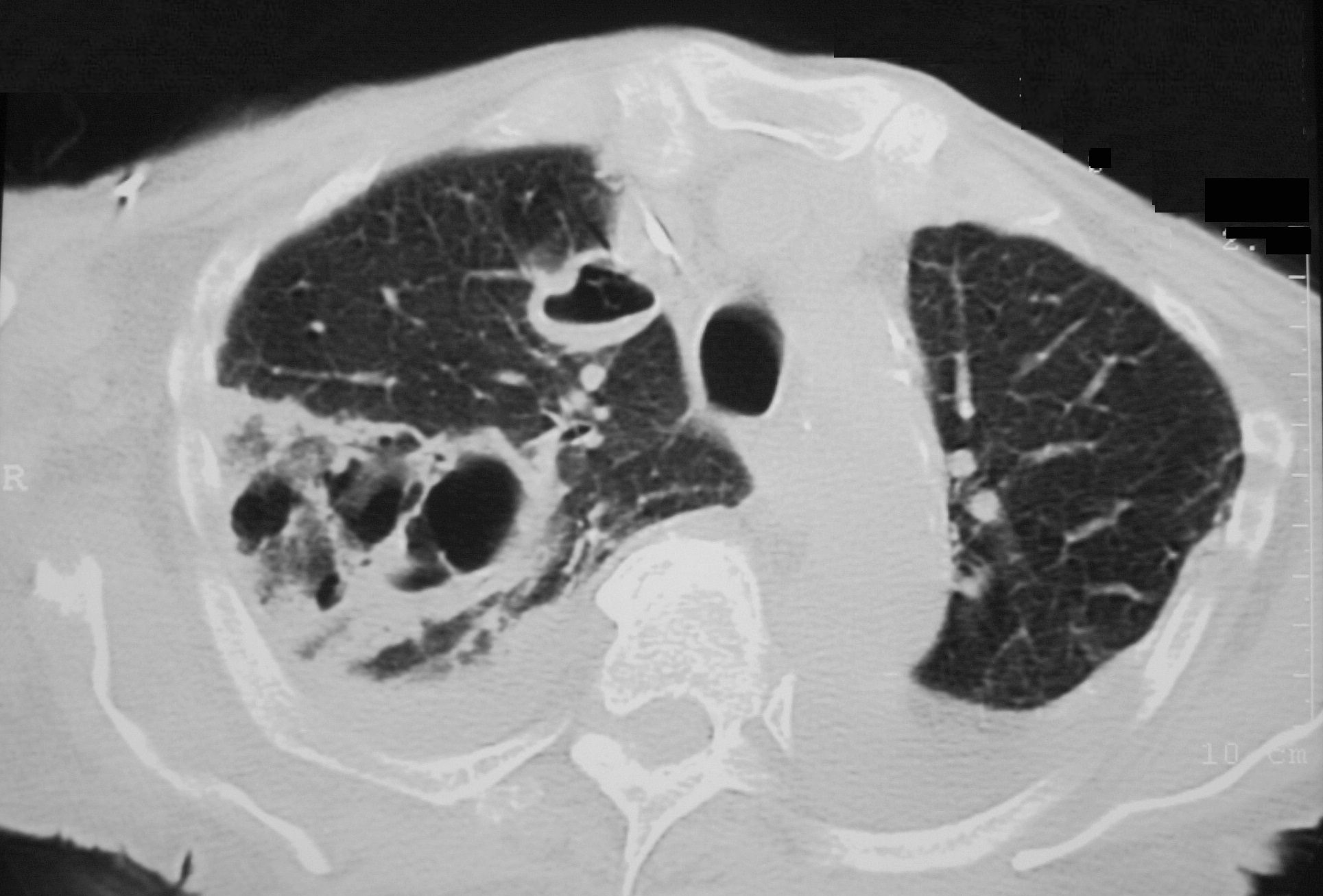
Axial CT reveals a large wedge shaped thick walled complex multicystic lesion associated with a feeding bronchovascular bundle (feeding vessel sign) in the right apex consistent with a cavitating infarction (cavitating Hampton’s hump). In addition there is a second smaller unilocular thick-walled cyst with a small air fluid level suggesting infection. There are pleural effusions. Echo showed tricuspid valve vegetations. Diagnosis is consistent with cavitating septic emboli
Ashley Davidoff TheCommonVein.net 33012 307Lu
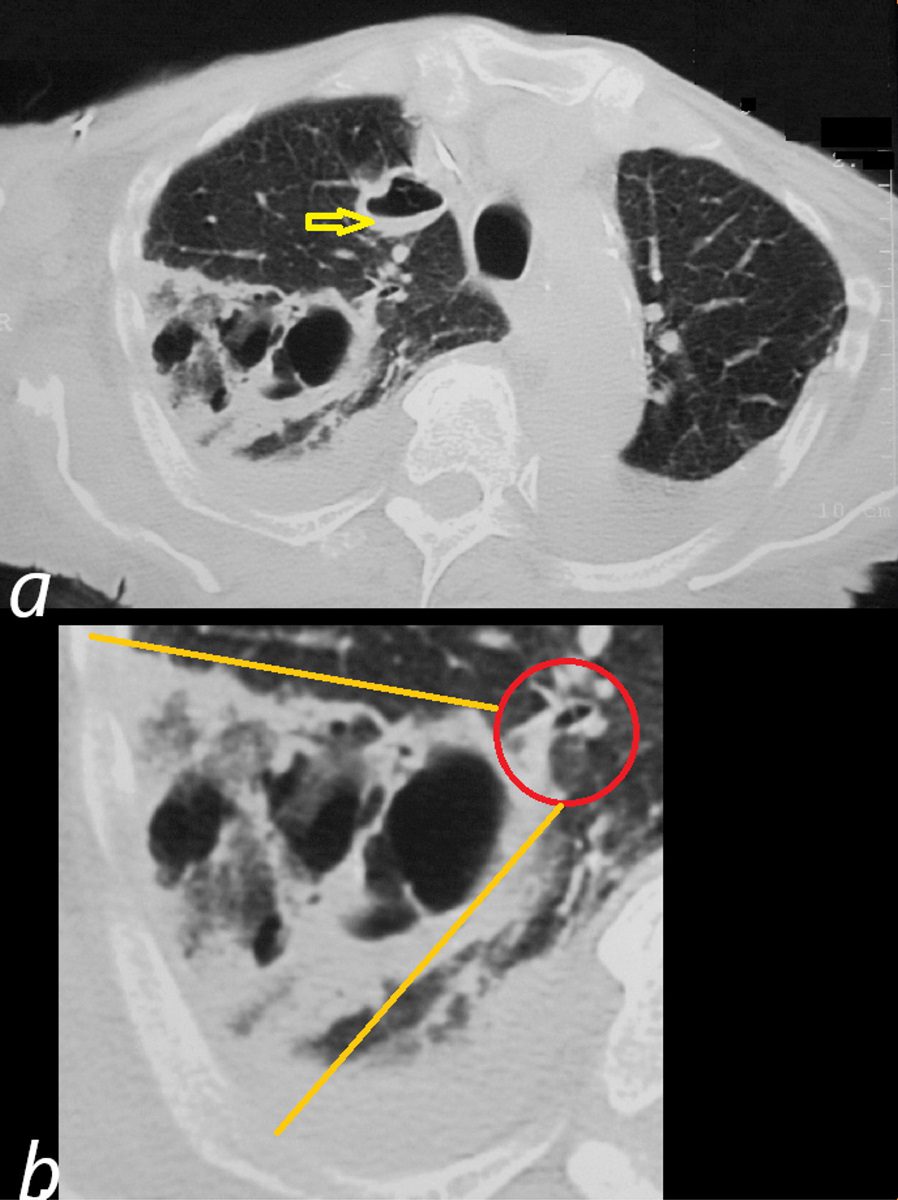
Axial CT reveals a large wedge shaped thick walled complex multicystic lesion ( bordered by orange lines in b) associated with a feeding bronchovascular bundle (red ring b -feeding vessel sign) in the right apex consistent with a cavitating infarction (cavitating Hampton’s hump). In addition there is a second smaller unilocular thick-walled cyst with a small air fluid level (yellow arrow, a) suggesting additional purulence in this clinical context. There are bilateral pleural effusions. Echo showed tricuspid valve vegetations. Diagnosis is consistent with cavitating septic emboli with pulmonary infarction.
Ashley Davidoff TheCommonVein.net 33012 307Lu
Prostate Seeds
Causes
Thromboembolic (most common):
Deep vein thrombosis (DVT).
Non-thrombotic (rare):
Fat embolism (e.g., long bone fractures).
Air embolism (e.g., surgery, trauma).
Amniotic fluid embolism (e.g., during labor).
Tumor embolism (e.g., metastatic cancers).
Risk Factors
Venous Stasis:
Prolonged immobility (e.g., bed rest, long flights).
Hypercoagulable States:
Genetic (e.g., Factor V Leiden, prothrombin mutation).
Acquired (e.g., cancer, pregnancy, oral contraceptive use).
Endothelial Injury:
Trauma, surgery, or indwelling catheters.
Clinical Presentation
Symptoms:
Sudden onset of dyspnea (shortness of breath).
Chest pain, often pleuritic.
Cough, sometimes with hemoptysis.
Signs:
Tachypnea (rapid breathing).
Tachycardia.
Hypoxia.
Hypotension (in massive PE).
Signs of DVT (e.g., swollen, painful leg).
Diagnosis
Imaging:
CT Pulmonary Angiography (CTPA):
Gold standard for diagnosis.
Shows filling defects in pulmonary arteries.
Ventilation-Perfusion (V/Q) Scan:
Used in cases where CTPA is contraindicated (e.g., pregnancy, renal impairment).
Ultrasound:
For DVT detection in the lower extremities.
Laboratory Tests:
D-dimer:
Elevated in PE but non-specific; useful for ruling out PE in low-risk patients.
Arterial Blood Gas (ABG):
Hypoxemia and respiratory alkalosis.
ECG:
May show signs of right heart strain (e.g., S1Q3T3 pattern, right axis deviation).
Echocardiography:
Evaluates right heart strain in massive PE.
Treatment
Anticoagulation:
First-line therapy to prevent further clot formation.
Heparin (unfractionated or low-molecular-weight) or direct oral anticoagulants (DOACs).
Thrombolysis:
For massive or high-risk PE with hemodynamic instability.
Surgical or Catheter-Based Thrombectomy:
In cases where thrombolysis is contraindicated or ineffective.
Supportive Care:
Oxygen therapy for hypoxia.
Hemodynamic support (e.g., fluids, vasopressors).
Complications
Chronic thromboembolic pulmonary hypertension (CTEPH).
Right heart failure.
Sudden death (in untreated or massive PE).
