Learning Objectives
define pulmonary embolism and deep vein thrombosis
discuss the pathogenesis and pathophysiology of the disorder including the risk factors
describe the characteristic and rare clinical presentations of pulmonary embolism
discuss the diagnostic tests used to diagnose pulmonary embolism
explain the general approach to management, prevention, complications and treatment
Introduction
Pulmonary embolism (PE) is defined as a circulatory disorder of the pulmonary arteries, characterized by embolic occlusion of one or more pulmonary arteries most commonly caused by venous thrombi in the legs.
PE is not a disease; rather, it is a complication of deep vein thrombosis (DVT). It is defined as a circulatory disorder of the pulmonary arteries, characterized by embolic occlusion of one or more pulmonary arteries by venous thrombi.
The clinical presentation is varied and ranges from the asymptomatic patient through chest pain and respiratory distress, to sudden collapse and sudden death.
The diagnosis is made by high clinical suspicion, blood tests, a Computed Tomography (CT) scan and treatment is with anticoagulation.
Background
Pulmonary embolism (PE) is a common and potentially lethal complication of DVT. In the United States there are approximately 700,000 cases a year accounting for up to 200,000 deaths per year. It is a true diagnostic dilemma on many levels and is often missed because patients with PE present with nonspecific signs and symptoms. If left untreated, approximately one third of patients who survive an initial PE, subsequently die from a future embolic episode. Most patients succumb to PE within the first few hours of the event. In patients who survive, recurrent embolism and death can be prevented with prompt diagnosis and therapy.
PE is a leading cause of morbidity and mortality particularly among hospitalized patients, with as many as 90% of pulmonary emboli being thrombotic. Thrombotic PE arises mostly from thrombi in the deep venous system of the lower extremity and less commonly from pelvic veins, upper extremity veins, right heart chambers or in situ. The remaining 10% of emboli arise from more rare causes including: air embolism, fat embolism, tumor, or amniotic fluid (1).
Pathogenesis and Pathophysiology
The coagulation cascade is an intrinsic protective mechanism, which is responsible for limiting blood loss by precisely regulating interactions between components of vessel wall, platelets and plasma proteins. However, unregulated activation of this cascade can have potentially deleterious consequences in the form of venous and arterial thrombosis, which may have life-threatening complications.
Rudolf Virchow, from autopsy studies on 11 cases of fatal pulmonary embolism in 1856, outlined a triad, which even till date forms the basis of our understanding of venous thrombosis.
The triad is composed of the following factors which contribute to formation of venous thrombosis, an endothelial lesion, venous stasis and a hypercoagulable state. Risk factors for venous thrombosis are operative by influencing one or all of the above three variables – by either the de novo development or an accentuation of preexisting elements of Virchow’s triad. Thus, trauma can result in immobility (venous stasis), tissue injury and its sequelae (hypercoagulability), and endothelial injury. Patients who already have a risk factor (e.g. hypercoagulability) may be more at risk for developing venous thromboembolism (VTE) after an event (e.g. surgery or trauma) than those who are free from predisposing factors. The presence of several risk factors in a patient results in a synergistic increase in the risk for VTE.

This diagram shows the triad of factors proposed by Virchow, that each or in combination can result in venous or arterial thrombosis. In this case venous thrombosis is shown with a normal vein and its valves shown to the left and thrombosis in a distended vein (light maroon) is shown to the right of the image.
Ashley Davidoff, M.D.

The venogram used to be the study of choice to rule out deep venous thrombosis until ultrasound was introduced in the 1990’s. Venography for DVT is rarely used today. However, this study shows in a graphic manner, the presence of clots in the deep veins of the calf and shows the tram tracking resulting from the contrast sliding up the sides of the thrombus implying that the clot is not attached to the walls, is loose, and is, therefore, easily detachable into the circulation with potentially devastating results. The clot is shown overlaid in maroon in (b) and (d). Once the clot is recognized, it is easier to go back to images (a) and (c) to identify the white column of contrast alongside the thrombus which gives the appearance of the tram-track.
Images courtesy of: Ashley Davidoff, M.D.
Pathogenesis and Pathophysiology Continued
Acute thrombus in the veins is minimally adherent to the wall of the vessel and hence the propensity to embolize to the right heart and then to the lungs resulting in a variable degree of occlusion of the pulmonary circulation. In general, the larger the thrombus load the greater the clinical consequence.
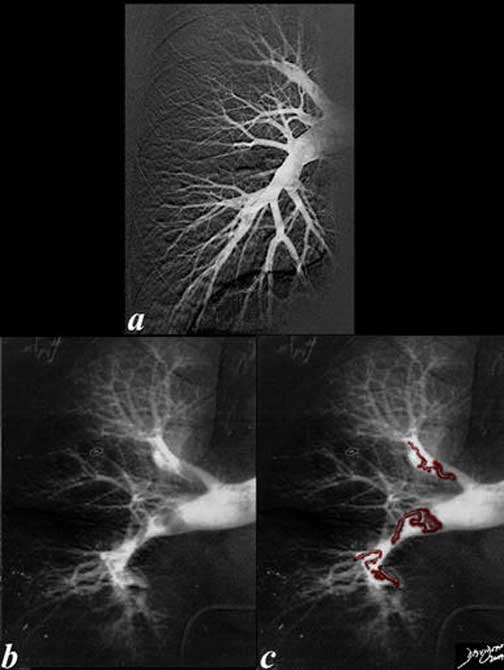
The angiograms of a right pulmonary artery are from a normal patient (a) and from a patient with pulmonary embolism (b) and (c). Note, the curled up linear thrombi in both the upper and lower pulmonary vessels that have a shape that is vaguely reminiscent of a cast of a vein. Once the thrombi are impacted or swirl in the circulation they become lobular, compacted, and rounded. Note, also the relative constriction of the distal vessels in the patient with embolism compared to the normal patient. The vasoconstriction is a known associated complication of PE.
Ashley Davidoff, M.D.
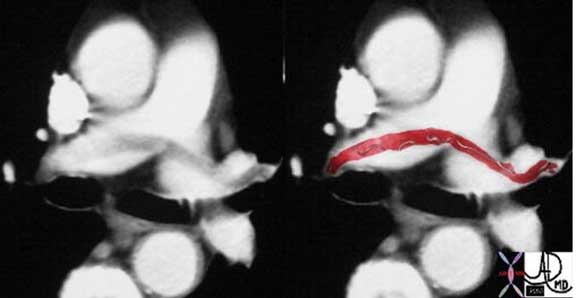
This case of a saddle embolus shows a thrombus sitting astride the left and right pulmonary arteries. Contemporary CT Angiography (CTA) is able to identify emboli in secondary and tertiary branches just as well. CTA has become the gold standard and study of choice in the patient with chest pain or acute desaturation with suspected PE.
Images courtesy of: Ashley Davidoff, M.D.
Risk Factors for Venous Thromboembolism
Strong risk factors (odds ratio >10
Fracture (hip or leg)
Hip or knee replacement
Major general surgery
Major trauma
Spinal cord injury
Moderate risk factors (odds ratio 2-9)
Arthroscopic knee surgery
Central venous lines
Chemotherapy
Congestive heart or respiratory failure
Hormone replacement therapy
Malignancy
Oral contraceptive therapy
Paralytic stroke
Pregnancy/postpartum
Previous venous thromboembolism
Thrombophilia
Weak risk factors (odds ratio <2)
Bed rest >3 days
Immobility due to sitting (e.g. prolonged car or air travel)
Increasing age
Laparoscopic surgery (e.g. cholecystectomy)
Obesity
Pregnancy/antepartum
Varicose veins
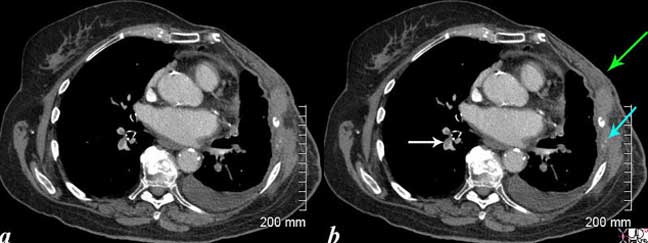
This CT scan is from an elderly lady with breast carcinoma, who presents with shortness-of-breath (SOB). Noted are filling defects in the pulmonary arteries (white arrow). The left-sided pulmonary arteries also have filling defects. She has had a left mastectomy (green arrow), and has lytic metastases of the left ribs with destruction of the bone which remain as two, white calcific specks (teal blue arrow). A small pleural effusion on the left may be due to the malignancy (likely) or due to the pulmonary embolus.
Courtesy of: Ashley Davidoff, M.D.
Pathology
Pulmonary embolism (PE) occurs when propagating clots break loose and embolize to lodge in the bifurcation of the main pulmonary artery or the lobar branches.
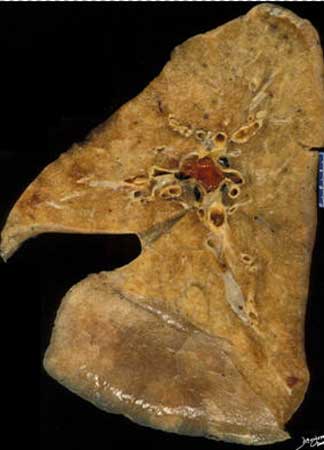
This is an autopsy specimen of the left lung in sagittal projection showing an occlusive thrombus in the main left pulmonary artery (red clot) extending into the lingula branch.
Most PE’s are small and clinically silent (60-80%). They eventually organize and get incorporated into the vessel wall or leave a delicate, bridging fibrous web. Embolic obstruction of small end arteriolar branches may cause infarction.
Ashley Davidoff, M.D.
Embolic obstruction of medium sized arteries may result in pulmonary hemorrhage but usually does not cause pulmonary infarction because of collateral bronchial artery flow. However, with left-sided cardiac failure and diminished bronchial circulation, infarcts can result.
Sudden death, right-sided heart failure or cardiovascular collapse occurs when 60% or more of the pulmonary circulation is obstructed with emboli.
The most specific finding related to acute PE at autopsy is a saddle thromboembolus at the main pulmonary artery or large coiled thromboemboli. The marks of the venous valves that certificated their embolic sources are sometimes imprinted on these thromboemboli.
White organized thrombi identified by the appearance of bands and webs serve as markers of latent past thromboemboli.

This postmortem specimen is of a coal miner who over 3 years developed increased opacity in the right apex (1, 2). At autopsy a wedge-shaped infarct was present (3, 4, 5, 6) with thrombus in the apical segmental artery(7, 😎 and areas of anthracotic pigment and massive fibrosis (9,10,11).
Images courtesy of: Ashley Davidoff, M.D.
Diagnosis
The clinical diagnosis of pulmonary embolism is difficult, particularly when there is coexisting heart or lung disease, and it is notoriously inaccurate when based on clinical signs alone. Very rarely, pulmonary embolism presents in such a dramatic fashion that the diagnosis is intuitively obvious and treatment will be started but the usual presentation is frequently vague and variable in severity, so that further testing is necessary to establish or exclude the diagnosis.
The first and most common presentation is dyspnea with or without pleuritic pain and hemoptysis (acute minor pulmonary embolism).
The second presentation is hemodynamic instability which is associated with acute massive pulmonary embolism.
The third and least common presentation mimics heart failure or indolent pneumonia, especially in the elderly (subacute massive pulmonary embolism).
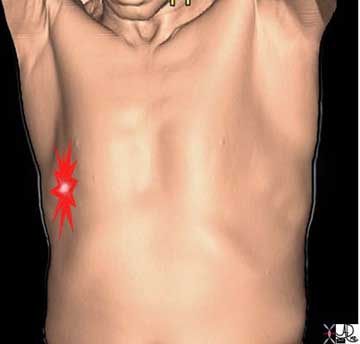
The pleuritic pain demonstrated on the right side of this patient is characterized by focal intense (bright red) and sharp pain that is aggravated by deep breaths and by coughing. The location corresponds to the disease and to the images described below.
Ashley Davidoff, M.D.
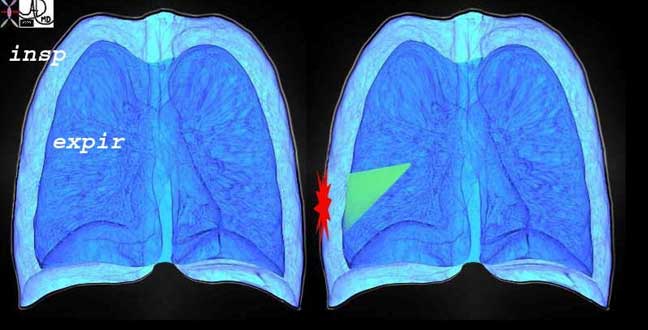
This diagram re-enforces the concept of the pleuritic pain being related to respiratory movements and is focal and overlying the disease process. The outer part of the diagram represents the expanded lung of inspiration and the inner less voluminous lung represents the deflated lung of expiration. The green wedge represents the infarcted area of lung from the pulmonary embolus that abuts and involves the visceral and parietal pleura.
Ashley Davidoff, M.D.
Diagnostic evaluation is best carried out by first attempting to identify a provable alternative diagnosis that can explain the patient’s symptoms. Most patients with pulmonary embolism will have one or more of the following clinical features:
dyspnea of sudden onset
tachypnea (> 20 breaths/minute)
or chest pain (pleuritic or substernal)
If the clinician remembers these three features, the possibility of pulmonary embolism will rarely be over-looked. By adopting a thorough stratification system the clinician can more appropriately select further investigations to prove or exclude pulmonary embolism. The most widely used and clinically relevant scoring system used is the modified Wells Criteria. This scoring system includes the following:
| Clinical Parameter Score | Score |
| Heart rate > 100 | +1.5 |
| Hemoptysis | 1 |
| Active cancer | +1 |
| Immobilization of the lower extremities (paralysis or recent fracture with cast) | +1.5 |
| Bedridden for >3 days or major surgery <4 weeks | +1 |
| Tenderness in Calf | +1 |
| Leg Edema | +1 |
| Calf swelling >3 cm compared with other asymptomatic leg | +1 |
| Asymmetric pitting edema (greater in the symptomatic leg) | +1 |
| Previous DVT or PE | +1.5 |
| Non varicose collateral superficial veins | +1 |
| Alternative diagnosis (as likely or greater than that of DVT) | -2 |
| Total of Above Score | |
| High probability | >3 |
| Moderate probability | 1 or 2 |
| Low probability | <0 |
Diagnosis: Acute Minor Pulmonary Embolism
If an embolus obstructs less than 50% of the pulmonary circulation, it often produces no symptoms. For example, about 40% of patients with DVT have no symptoms of pulmonary embolism but do have evidence of the condition on lung scans. If symptoms do develop the most common is dyspnea, possibly upon minor exertion. Sometimes, the first abnormality the patient notes results from pulmonary infarction which occurs in obstruction of medium-sized pulmonary artery branches. Sharp pleuritic pain develops and there may be associated hemoptysis. Pulmonary infarction occurs in only about 10% of patients without pre-existing cardiopulmonary disease. If, however, there is already compromise of the oxygenation of the embolized area, either because the airways are abnormal or pulmonary venous outflow is impaired as a result of pre-existing left heart disease, then the incidence of infarction rises to 30%. If there are any physical signs, they are those of pulmonary infarction. The patient is distressed with rapid and shallow breathing because of the pleuritic pain, but is not cyanosed because the disturbance of gas transfer is only slight. Signs of pulmonary infarction may be found in the lungs, a mixture of consolidation and effusion, possibly with a pleural rub. Fever is common and sometimes differentiation from infective pleurisy is difficult. The fever and pain often produce a sinus tachycardia. Pulmonary artery mean pressure rarely exceeds 25 mm Hg. As minor pulmonary embolism does not compromise the right ventricle, cardiac output is well maintained.
Diagnosis: Acute Massive Pulmonary Embolism
When more than 50% of the pulmonary circulation is suddenly obstructed, the pathophysiology and clinical signs become dominated by the severe derangement of cardiac and pulmonary function. Obstruction of the pulmonary artery and mediator induced vasoconstriction causes a substantial increase in right ventricular afterload and, if the cardiac output is to be maintained, consequent elevation of pulmonary artery systolic pressure and an increase in right ventricular work. If this work cannot be sustained, acute right heart failure occurs. The thin walled right ventricle is designed to work against the normally low pulmonary vascular resistance; it performs poorly against a sudden obstruction. As a result, it dilates, and a moderate rise in the right ventricular and pulmonary artery systolic pressure occurs, which rarely exceeds 55 mm Hg because the ventricle, lacking time to develop compensatory hypertrophy, is unable to generate a higher pressure. The right ventricular end diastolic pressure and right atrial pressure rise to about 15-20 mm Hg as the ventricle fails. Right ventricular dilatation leads to tricuspid regurgitation and may compromise the filling of the left ventricle. Cardiac output falls and the patient becomes hypotensive. This may occur so rapidly that syncope is either the presenting feature or easily induced by a relatively minor cardiovascular stress. If the degree of obstruction is sufficient, death occurs almost immediately. The fall in aortic pressure and the rise in right ventricular pressure may cause ischemia of the right ventricle through a critical reduction of right coronary perfusion. Electro-mechanical dissociation is the most frequent cause of final cardiac arrest.

A 43-year-old -male presented with sudden cardiovascular collapse. The CTA shows a saddle embolus with total impaction of the thrombus in the right main pulmonary artery and impaction to lesser extent in the left pulmonary artery.
Ashley Davidoff, M.D.

The series of CT scans are from an elderly lady who presents with chest pain, right leg pain and severe cardiorespiratory distress. The CTA shows massive pulmonary embolism to the right main pulmonary artery (maroon overlay in b) and evidence of an infarction (white arrow). There is dense contrast in the dilated hepatic veins while we are still in the arterial phase of the injection (blue arrow). This means that contrast is refluxing from the right atrium deep into the hepatic veins and this represents CT evidence of tricuspid regurgitation and right ventricular strain and pressure overload.
Images courtesy of: Ashley Davidoff, M.D.

The angiogram is from a young man who presented with chest pain, acute respiratory distress and near cardiovascular collapse. A pulmonary angiogram (a, b) shows massive extensive small pulmonary emboli characterized by cut offs in all the visualized vessels (arrows) with severe and unusual pulmonary vasoconstriction. These changes could have been due to pulmonary emboli that precipitated a neurohormonal response causing severe vasoconstriction. An alternate explanation for these findings may be that there was primary vasoconstriction, with secondary thrombosis due to slow flow. Either way the combination of vasoconstriction and pulmonary emboli is a devastating combination. Image (c) shows a different patient who has a normal circulation. He was treated with intrapulmonary urokinase infusion through the catheter and he responded with some return of blood flow to the lungs shown later in the module. Image courtesy of: Ashley Davidoff, M.D.
Arterial hypoxemia correlates roughly with the extent of embolism if there is no prior cardiopulmonary disease. Massive pulmonary embolism without hypoxemia is so rare that if the arterial oxygen tension (PaO2) is normal an alternative diagnosis should be considered. The main causes of hypoxemia in pulmonary embolism seem to be as follows:
(1) Ventilation-perfusion mismatch. Non-embolized areas of the lung are relatively overperfused, so that the ventilation in these areas may be insufficient to oxygenate fully the extra blood flow.
(2) Shunting occurs through areas of collapse and infarction that are not ventilated but retain some blood flow. This may become more important a few days after the initial episode. In patients with a patent foramen ovale, raised right atrial pressure may open the foramen and cause right-to-left shunting at the atrial level. This should be considered if the degree of hypoxemia is more profound than would be expected from other clinical features, if it cannot be corrected by oxygen administration and if it is accompanied by hypercapnia.
(3) Low mixed venous oxygen saturation caused by the reduced cardiac output causes hypoxemia because there is insufficient time for the extremely desaturated blood to become fully saturated as it passes through the alveolar capillaries in the over-perfused areas of the lung.
Although pulmonary embolism impairs the elimination of CO2, hypercapnia is rare because compensatory hyper ventilation eliminates CO2 in all but the most extensive embolism. In cases with a sufficient degree of vascular obstruction to produce hypercapnia, the hemodynamic sequelae of acute right ventricular failure usually prove fatal.
The clinical features of acute massive pulmonary embolism can be explained in terms of these pathophysiological changes. The patient becomes acutely distressed, severely short of breath, and may be syncopal because of the combination of hypoxemia and low cardiac output. The combination of hypotension, hypoxemia, and increased cardiac work may cause anginal chest pain. The physical signs are those of reduced cardiac output, that is, pronounced sinus tachycardia, hypotension, and a cool periphery, sometimes with confusion. The patient is obviously dyspneic (but not orthopneic), cyanosed both centrally and peripherally, and has signs of acute right heart strain: a raised venous pressure, which is often difficult to appreciate because of the respiratory distress, a gallop rhythm at lower sternum and a widely split second heart sound due to delayed right ventricular ejection, which is difficult to detect because of the accompanying tachycardia. The pulmonary component of the second heart sound is usually not loud because the pulmonary artery pressure is only moderately raised.
Diagnosis: Subacute Massive Pulmonary Embolism
Subacute massive pulmonary embolism is caused by multiple small or moderately sized emboli that accumulate over several weeks. Because the obstruction occurs slowly, there is time for the right ventricle to adapt and for some hypertrophy to develop; consequently, the right ventricular systolic pressure is higher than in acute pulmonary embolism. The rises in the right ventricular end diastolic and right atrial pressures are of a lesser extent than in acute massive pulmonary embolism since there is time for adaptation to occur and the degree of right ventricular failure is less for a given degree of pulmonary artery obstruction. The main symptoms are increasing dyspnea and falling exercise tolerance. There is often an associated dry cough. The breathlessness is usually out of proportion to all other findings and there may be central cyanosis. The blood pressure and pulse rate are usually normal because the cardiac output is well maintained. Commonly, the venous pressure is raised and a third heart sound is audible at the lower sternum which may be accentuated by inspiration. The pulmonary component of the second heart sound is sometimes loud. There may also be intermittent symptoms and signs of pulmonary infarction that occurred during the build up of the obstruction. In advanced cases, cardiac output falls and frank right heart failure develops. A further pulmonary embolism may change the picture to that resembling acute massive pulmonary embolism.
Diagnosis: CT Pulmonary Angiography
The imaging study of choice for pulmonary embolism is CT pulmonary angiography (CTPA). Conventional pulmonary angiography was known as the gold standard, but has been surpassed by CTPA and now is only used for selected cases. CT pulmonary angiography is the most rapid, sensitive and accurate way to diagnose pulmonary emboli. Criteria for a positive CT scan result include a partial filling defect (defined as intraluminal area of low attenuation surrounded by a contrast medium), a complete filling defect, and the “railway track sign” (masses seen floating in the lumen, allowing the flow of blood between the vessel wall and the embolus). The procedure has over 90% specificity and sensitivity in diagnosing pulmonary embolism in the main, lobar, and segmental pulmonary arteries. The diagnostic accuracy can reach as high as 96% with a high clinical likelihood (5). However, this percentage can vary on the quality of images, experience of the radiologist and the patient’s pulmonary anatomy. One major advantage of this imaging is its capability to identify other causes that may account for the patient’s presenting symptoms. Cautions with this modality include patients at risk for adverse effect for IV contrast, such as patients with chronic kidney disease or acute renal failure as indicated by an increased creatine level. Some patients are allergic to IV contrast. Pregnant patients in the first 14 weeks of pregnancy should not be subjected to the radiation.

This CT demonstrates massive pulmonary embolism with compaction of the embolus in the main pulmonary arteries. The digital technology allows for reconstruction of the images in any plane enabling the evaluation of the extent of the thrombus. The axial images have been reconstructed in the sagittal plane in (a) and (b).
Ashley Davidoff, M.D.
The CT technology is rapid in the acquisition of the images and also allows the images to be manipulated and reconstructed in any plane providing considerable advantages over nuclear medicine and conventional angiography.
Timing of the bolus is absolutely essential for diagnostic accuracy. Timing infers that the peak concentration of the contrast should be present in the pulmonary arteries when the images are taken. Each patient has different hemodynamics and so a variety of electronic density sensors to enable accuracy have been developed to aid in the bolus timing.
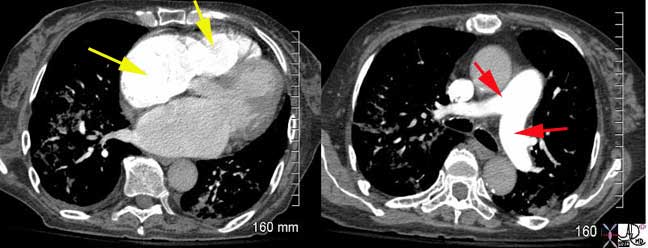
This is a CT pulmonary angiogram (CTPA) of an elderly female who presented with hypoxia and PE was included in the differential diagnosis. The bolus in this instance is perfectly timed with the right chambers optimally and maximally enhanced, right atrium and right ventricle (yellow arrows) in the left image and the pulmonary arteries (red arrows) optimally enhanced in the right image. The left atrium and left ventricle in the image on the left, and ascending aorta and descending in the image on the right, are almost devoid of the contrast. This is a perfectly timed bolus. No pulmonary emboli were seen but the parenchymal infiltrates at both bases account for her presentation. Images courtesy of: Ashley Davidoff, M.D.
Diagnosis: Chest Radiography
Chest radiographic findings are also non-specific but may be helpful. A normal examination is compatible with all types of acute pulmonary embolism; in fact, a normal film in a patient with severe acute dyspnea without wheezing is very suspicious of pulmonary embolism. The lung fields may show evidence of pulmonary infarction, peripheral opacities, sometimes wedge-shaped or semicircular, arranged along the pleural surface (so called Hampton’s hump). It may be possible to detect areas of oligemia in the parts of the lung affected by emboli (Westermark sign). Similar to the ECG the radiograph is especially valuable in excluding other conditions mimicking pulmonary embolism.
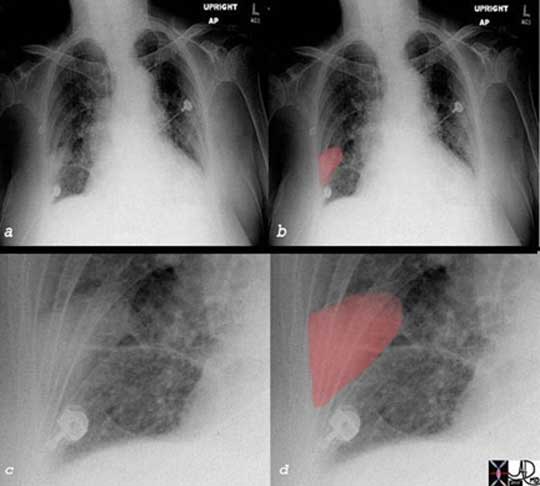
The plain film of a patient with right-sided pleuritic pain and hypoxemia is shown. Image (a) shows a vague wedged-shaped defect in the right lower lobe [overlaid in red in (b), enlarged in (c) and (d)]. This wedge shaped-peripheral based defect is known as Hampton’s hump and is suggestive of a pulmonary infarction, and subsequently proven by CT scan. When a pulmonary embolism causes an infarction, it may be seen as a wedged-shaped defect on the chest x-ray. However, it is not a very common event since the lung has three sources of oxygen: pulmonary artery, bronchial artery and directly from the inspired air to the alveoli Since the pulmonary circulation of the lung is an end arterial circulation, the most distal portion of the segment of the lung and its pleura will be affected when infarction does occur. The accompanying inflammatory process will involve the pleura and pleuritic pain ensues..
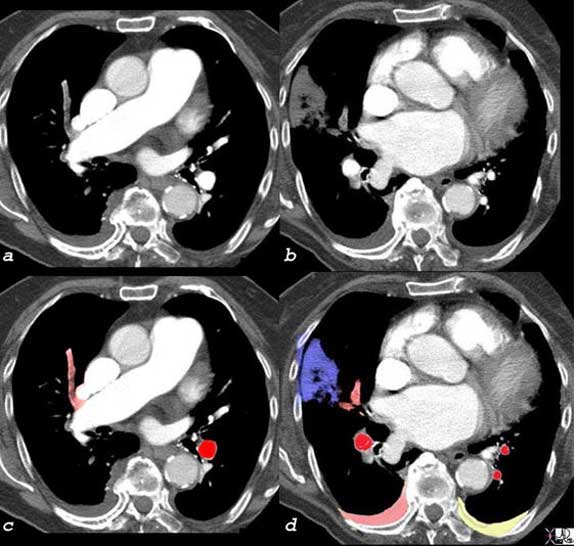
The CT scan of this patient shows an occlusive embolus [gray in (a) overlaid in pale red in (c)] as well as the branches of this vessel [gray in (b)] and overlaid in pale red in (d) resulting in a peripheral pleural based infarction [gray in (b)] and overlaid in blue in (d) there are bilateral effusions with right effusion (pink overlay) likely to be hemorrhagic and the left effusion likely to be transudative. Pulmonary infarction as seen in this case is not common since the lung receives oxygen directly from the alveoli, as well as from the bronchial arterial circulation. However, in this patient there was associated congestive heart failure and perfusion and ventilation were reduced at base line. When one adds insult to injury with a PE, compensatory mechanisms are not available and infarction ensues.
Ashley Davidoff, M.D.

The images include a plain film and a CT of a 61-year-male with a diagnosis of non small cell carcinoma treated 9 months prior and a recent admission to an outside institution with MRSA. He was transferred to current hospital due to worsening blood gases and CTA showed complete occlusion of the left pulmonary artery. Doppler showed DVT of the right popliteal vein. He had a subsequent episode of worsening of blood gases and a chest X-ray showed a complete white out of the right lung and a mid region (upper triangle in lower lung zone in (b)) of a paucity of blood vessels. This is known as Westermark’s sign.
The white out on the right side was thought to be due to aspiration. He thus had no ventilation of the right lung and almost no perfusion of the left lung. He was bronchoscoped and thick secretions were removed from the right bronchus. Despite treatment on anticoagulants and repeated bronchoscopies the patient unfortunately expired.
Images courtesy of: Ashley Davidoff, M.D.
Other Diagnostic Tests
ECG
ECG changes are usually non-specific. In minor pulmonary embolism there is no hemodynamic stress and thus the only finding is sinus tachycardia. In acute or subacute massive pulmonary embolism, evidence of right heart strain may be seen (rightward shift of the QRS axis, transient right bundle branch block, T-wave inversion in leads V1-3, P pulmonale), but the classic SIQIIITIII pattern occurs in only a few cases.(2) The main value of ECG is in excluding other potential diagnoses, such as myocardial infarction or pericarditis.
Arterial Blood Gas
The main utility is looking for hypoxia and an elevated alveolar-arterial gradient. The PaO2 is almost never normal in the patient with massive pulmonary embolism, but can be normal in minor pulmonary embolism, mainly due to hyperventilation. In such cases the widening of the alveolo-arterial PO2 gradient (AaPO2 > 20 mm Hg) may be more sensitive than PaO2 alone.
Echocardiography
Transthoracic echocardiography rarely enables direct visualization of the pulmonary embolus but may reveal thrombus floating in the right atrium or ventricle.. In massive pulmonary embolism the right ventricle is dilated and hypokinetic, with abnormal motion of the interventricular septum. The inferior vena cava does not collapse during inspiration. There is some evidence that regional right ventricular dysfunction (akinesia of the mid-free wall with apical sparing) may be more common in acute pulmonary embolism. The Doppler technique allows the pulmonary artery pressure to be estimated and together with contrast echocardiography it is useful in diagnosing patent foramen ovale which may indicate impending paradoxical embolism.
D-Dimer
A D-Dimer is fibrin degradation product found in the blood. In pulmonary emboli or any thrombosis, this level is raised. The D-Dimer is sensitive but not specific, which makes it a test with good negative predictive value. Patients that have a normal D-Dimer level have a 95% likelihood that they do not have an acute pulmonary embolism. This percentage increases to 99% if there is a low clinical probability for embolus, such as, when the Wells score is <2 . In elderly or inpatients, D-dimer retains a high negative predictive value, but is normal in less than 10% of patients and hence, not very useful.
V/Q Scan
The V/Q scan is a nuclear medicine modality where Technetium labeled albumin is used to identify ventilation-perfusion (V/Q) mismatches in the lung. A normal perfusion scan essentially excludes the diagnosis of a clinically relevant recent pulmonary embolism because occlusive pulmonary embolism of all types produces a defect of perfusion. Normal results are almost never associated with recurrent pulmonary embolism, even if anticoagulants are withheld. However, many conditions other than pulmonary embolism, such as tumors, consolidation, left heart failure, bullous lesions, lung fibrosis, and obstructive airways disease, can also produce perfusion defects. Addition of a ventilation scan increases the specificity of scintigraphy. Pulmonary embolism usually produces a defect of perfusion but not ventilation (“mismatch”) while most of the other conditions produce a ventilation defect in the same area as the perfusion defect (matched defects). Pulmonary embolism can also produce matched defects when infarction has occurred, but in this situation the chest radiograph nearly always shows an abnormality in the area of scan defect.
These mismatches when read by an experienced radiologist can be stratified into low, intermediate, and high probability for pulmonary embolism. The landmark Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study, investigated the accuracy of V/Q scans as compared to the gold standard for diagnosis, pulmonary angiogram. The study found that patients with a high clinical suspicion for embolus and a high probability V/Q scan had a 95% chance of having an acute pulmonary embolism.
Scanning should be performed within 24 hours of the onset of symptoms of pulmonary embolism, since some scans revert to normal quickly.
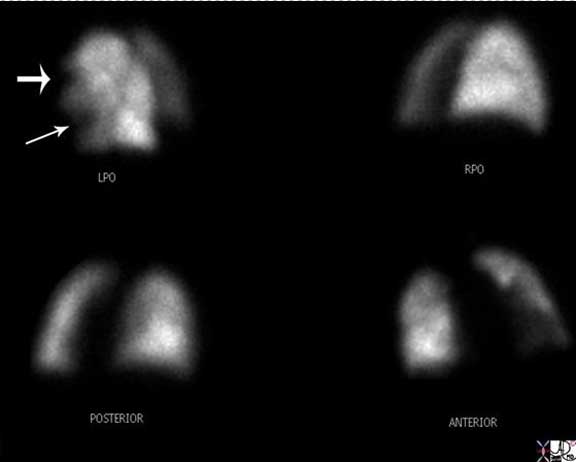
This is the perfusion component of a ventilation perfusion nuclear medicine scan, in an 81-year-old female with lymphoma who presents with shortness of breath. Segmental defects are noted (arrows) in the lingula and the anterior upper lobe segment of the left lobe . The chest X-ray was normal indicating that there were no other pulmonary causes for the defects. These finding are therefore indicative of a high probability for PE.
Image courtesy of: Ashley Davidoff, M.D.
Although the lung scan is often an imprecise guide it is useful in clinical decision making; a normal scan or a low probability scan with low clinical likelihood of pulmonary embolism means that treatment for suspected pulmonary embolism can be withheld and a high probability scan with a high clinical likelihood of pulmonary embolism means that treatment is mandatory.
Potential Complications
The potential complications of a pulmonary embolism are related to the size and location of the embolus. In the case of a massive acute embolism, sudden cardiac death is possible. Acute cor pulmonale (acute right ventricular failure) is also a result of a massive pulmonary embolism, usually obstructing greater than 60-75% of the pulmonary arterial circulation or the embolus is a “saddle embolus,” at the bifurcation of the pulmonary artery. Cor pulmonale can lead to hypotension and cardiac arrest if not treated promptly. Pulmonary infarction may be a complication in patients with a sub massive embolism that completely obstructs a distal arterial branch. Hemoptysis will be a clue to this process. If the acute pulmonary embolism is non-fatal and goes undiagnosed, an entity called chronic thromboembolic pulmonary hypertension may occur. This entity may present years after diagnosis, presenting with signs of pulmonary hypertension.
Management
The aim of treatment for PE is to relieve symptoms, prevent death, reduce the risk of developing chronic pulmonary hypertension, and prevent recurrence. Treatment is often based on the clinical probability of pulmonary embolism rather than on a definite diagnosis or a ruling out of the condition. Consequently, some patients receive anticoagulants without proof of pulmonary embolism and other patients are not treated although they may have it. Anticoagulation is the mainstay of treatment for PE. The role of pharmacological or mechanical embolectomy is restricted to patients who are hemodynamically unstable at presentation.
Anticoagulation
When anticoagulation is begun, the choices usually include low molecular weight heparin or unfractionated heparin. Early heparin anticoagulation is so essential that heparin should be started as soon as the diagnosis of significant pulmonary thromboembolism is considered. Heparin reduces the mortality rate of PE because it slows or prevents clot progression and reduces the risk of further embolism by augmenting the activity of antithrombin III and preventing the conversion of fibrinogen to fibrin. Heparin does nothing to dissolve the clot that has developed already but it is still the single most important treatment that can be provided because the greatest contribution to the mortality rate is the ongoing embolization of new thrombi. Prompt effective anticoagulation has been shown to reduce the overall mortality rate from 30% to less than 10%.
With proper dosing, several [low molecular weight heparin (LMWH)] products have been found safer and more effective than unfractionated heparin both for prophylaxis and for treatment of DVT and PE. Monitoring the aPTT is neither necessary nor useful when giving LMWH, because the drug is most active in a tissue phase and does not exert most of its effects on coagulation factor IIa. Enoxaparin and tinzaparin are currently approved by the FDA for treatment of DVT. Dalteparin is FDA approved for prophylaxis and has approval for cancer patients.
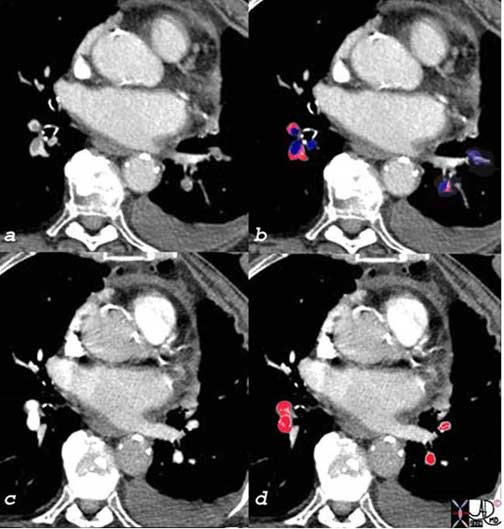
The patient has breast carcinoma and was shown in the set of images above. She presented with shortness of breath and pulmonary emboli were diagnosed as filling defects [overlay in royal blue (c)], in all the visualized vessels. After two weeks of anticoagulant therapy the emboli were lysed and the vessels were completely patent [overlaid in red in (d)].
Courtesy of: Ashley Davidoff, M.D.
Fibrinolytic Therapy
Fibrinolytic therapy should be considered for three groups of patients, unless overwhelming contraindications are evident:
those who are hemodynamically unstable
those with right heart strain and exhausted cardiopulmonary reserves
those who are expected to have multiple recurrences of pulmonary thromboembolism over a period of years.
Patients with a prior history of PE and those with known deficiencies of protein C, protein S, or antithrombin III should be included in this latter group.
Fibrinolytic regimens currently in common use for PE include two forms of recombinant tissue plasminogen activator, t-PA (alteplase) and r-PA (reteplase), along with urokinase and streptokinase. Alteplase usually is given as a front-loaded infusion over 90 or 120 minutes. Urokinase and streptokinase usually are given as infusions over 24 hours or more. Reteplase is a new-generation thrombolytic with a longer half-life that is given as a single bolus or as 2 boluses administered 30 minutes apart. Of the 4, the faster-acting agents, reteplase and alteplase are preferred for patients with PE, because the condition of patients with PE can deteriorate extremely rapidly.
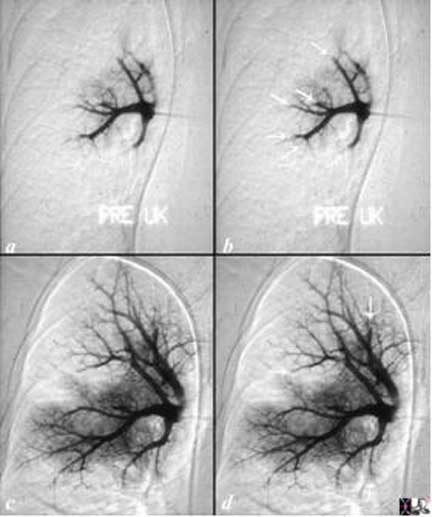
The angiogram is from a young man who presented with acute respiratory distress and chest pain. A pulmonary angiogram (a, b) showed severe pulmonary vasoconstriction and cut off of multiple vessels consistent with thrombosis (white arrows (d). These could have been pulmonary that precipitated a neurohormonal response causing severe vasoconstriction, or the vasoconstriction may have been primary with secondary thrombosis due to slow flow. He was given urokinase infusion through the catheter and he responded with some return of blood flow to the lungs but with residual filling defects (c) and arrows in (d) but with still significant vasoconstriction. Urokinase is no longer used but was partially successful when used in this patient to lyse the clot. Courtesy of: Ashley Davidoff, M.D.
Prevention of Recurrence
Long-term anticoagulation is essential for patients who survive an initial DVT or PE. The optimum total duration of anticoagulation has been controversial in recent years, but general consensus holds that at least 6 months of anticoagulation is associated with significant reduction in recurrences and a net positive benefit. Warfarin is dosed to achieve an INR between 2 and 3. When the pulmonary embolus is the patient’s first, and the patient has an identifiable cause, the warfarin therapy is continued for 3-6 months. If the patient has recurrent emboli, therapy is extended from 6-12 months to possibly life-long depending on the cause.
Case Studies
Now we will look at a few interesting and unusual cases of pulmonary emboli.
Embolization of Venous Thrombosis of the Upper Limbs
Upper limb venous thrombosis is common because the upper limbs and superior vena cava are used so frequently for venous access. Symptomatic embolization from the upper limbs is quite uncommon.
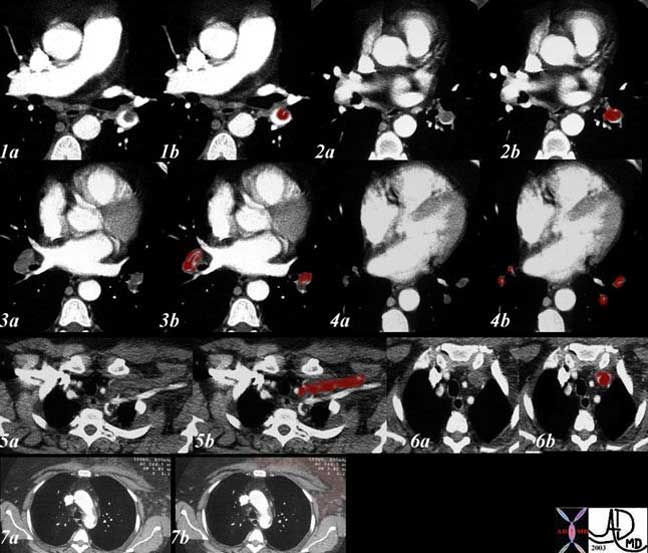
This elderly woman presented with acute respiratory difficulty with swelling of her left upper limb. CT shows extensive pulmonary embolic disease to the lower lobes from second order branches to the tertiary branches (1-4 with maroon overlay) and evidence of thrombosis of the left subclavian vein. (5, 6). Swelling over the anterior chest including the pectoralis muscles is evident in (7) caused by the venous obstruction.
Ashley Davidoff, M.D.
Pulmonary Emboli and Systemic Emboli in the Same Patient
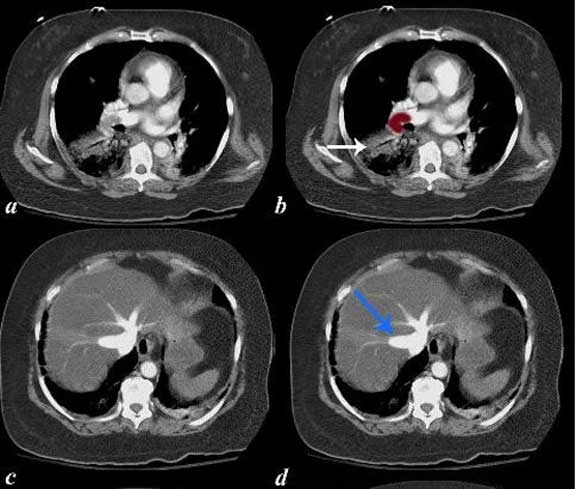
The series of CT scans are from an elderly lady who presented with chest pain and right leg pain. Images (a) and (b) show a large pulmonary embolus to the right pulmonary artery and an infiltrate in the right lower lobe compatible with a pulmonary infarct. Pulmonary hypertension secondary to the embolus resulted in strain on the right ventricle and in images (c) and (d), tricuspid regurgitation is noted as a consequence characterized by hepatic vein regurgitation (blue arrow). This scenario was presented above.
In addition

Embolization to the right femoral artery (g, h) was also apparent.
A bubble echocardiogram showed a patent foramen ovale. The scenario, therefore, was initiated by a pulmonary embolism with secondary elevation of right sided pressures which created a right to left shunt and embolization to the systemic circulation. Images courtesy of: Ashley Davidoff, M.D.
Septic Emboli
Septic emboli are an insidious infection of the lung, originating from the venous system, or right side of the heart. Causes include vegetations of the tricuspid valve, (intravenous drug abusers), infected right-sided catheters, pelvic thrombophlebitis, peripheral venous thrombophlebitis (heroin drug abusers), cellulitis and skin carbuncles. The organisms are commonly staphylococcus aureus and streptococcus.
Presenting symptoms include sepsis, shaking chills, cough, dyspnea, hemoptysis, and chest pain.
Diagnosis is based on blood cultures and imaging studies that include echocardiography, chest X-ray, and CT of the chest. Cavitating multicentric nodules with a predilection for the lung bases are characteristic. Subsegmental wedge-shaped infarcts are noted as well, and sometimes a feeding vessel is seen subtending the nodules. Treatment is with antibiotics.
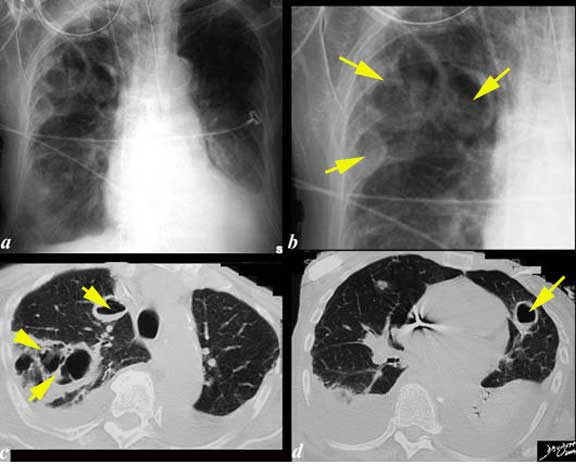
The chest x-ray (CXR) and CT scan are from a 76-year-old patient who had a complicated history of septicemia and bacterial endocarditis of the mitral valve associated with a septic arthritis and an infected ICD wire. She has a previous medical history of MI, ischemic cardiomyopathy with an ejection fraction of 20%, PTCA of LAD, RCA stent, chronic renal failure and COPD. Her WBC was 33 thousand; Staphylococcus aureus was isolated from her sputum. The CXR shows multiple large cavitating lesions with three large lesions in the right upper lobe (a, b, c), one in the right lower lobe (a) and one large lesion alongside the heart in the lingula (b, d). These findings are consistent with septic emboli with cavitation.
Images courtesy of: Ashley Davidoff, M.D.
Fat Embolism
Fat embolism syndrome (FES) has been described as occurring after traumatic, surgical, and atraumatic conditions. It most commonly occurs after lower extremity trauma and intramedullary surgery and can result in a multi-system manifestation of embolization when fat droplets act as emboli, becoming impacted in different organ microvasculature and microvascular beds, causing damage to the small vessels. Although FES usually presents as a multisystem disorder, the most seriously affected organs are the lung, brain, cardiovascular system, and skin.
Up to 75% of patients with FES present with some degree of respiratory failure, ranging from nearly asymptomatic hypoxemia to pulmonary distress requiring ventilatory support. An asymptomatic latent period of about 12-48h precedes the clinical manifestations. Patients become tachypneic, dyspneic, and hypoxic as a result of ventilation-perfusion abnormalities 12-72h after trauma. The fulminant form of FES presents as acute cor pulmonale with respiratory failure, resulting in death within a few hours of injury. Fat embolism syndrome is a self-limiting disease and treatment should be mainly supportive.

Shown are images from a 21-year-old college student and football player who sustained a compound fracture of his right tibia and fibula. While awaiting surgery he became short of breath, tachypneic and tachycardic and his saturations dropped to 70’s from 90’s on room air. He had a fever to 103, heart rate of 100, respiratory rate of 30, and a systolic pressure of 140. He required intubation for 5 days but did recover thereafter. These findings are consistent with fat embolism: the history, type of injury, the clinical presentation and the lack of thrombotic emboli.
Courtesy of: Ashley Davidoff, M.D.
Air Embolism
Venous air embolism (VAE), the entry of gas into the peripheral or central vasculature, can occur secondary to iatrogenic complications, trauma, and even certain recreational activities.
Gas emboli are usually composed of air but they can also occur with medically used gases such as carbon dioxide, nitrous oxide, and nitrogen. Although very small volumes of air can lead to severe sequelae, it is generally accepted that more than 50 mL of air can cause hypotension and dysrhythmias and more than 300 mL of air can be lethal.
Transesophageal echocardiography and CT scan are the most sensitive monitoring modalities for VAE and can detect as little as 0.02 mL/kg of air. Precordial Doppler ultrasonography is also an effective monitoring technique and can detect as little as 0.25 mL of air.
Treatment should begin with:
1. the administration of 100% oxygen and intubation for significant respiratory distress or refractory hypoxemia. Oxygen may reduce bubble size by increasing the gradient for nitrogen to move out.
2. prompt placement of the patient in Trendelenburg (head down) position and rotating toward the left lateral decubitus position. This maneuver helps trap air in the apex of the ventricle, prevents its ejection into the pulmonary arterial system, and maintains right ventricular output.
3. maintaining systemic arterial pressure with fluid resuscitation and vasopressors/beta-adrenergic agents if necessary.
4. hyperbaric chambers may be considered.
Amniotic Fluid Embolism
Amniotic fluid embolism (AFE) is a rare obstetric emergency in which it is postulated that amniotic fluid, fetal cells, hair, or other debris enter the maternal circulation, causing cardiorespiratory collapse. The diagnosis of AFE has traditionally been made at autopsy when fetal squamous cells are found in the maternal pulmonary circulation; however, fetal squamous cells are commonly found in the circulation of laboring patients who do not develop the syndrome. In a patient who is critically ill, a sample obtained by aspiration of the distal port of a pulmonary artery catheter that contains fetal squamous cells is considered suggestive of but not diagnostic of AFE syndrome. The diagnosis is essentially one of exclusion based on clinical presentation. Other causes of hemodynamic instability should not be neglected. Treatment is supportive.
Tumor Embolization
Certain tumors have a predilection to invade the veins, either through normal venules or the venules that are part of the neovascularity of the tumor. Once they are in the veins they are taken to the pulmonary circulation where they become trapped. If they do not proliferate and spread locally they are considered as emboli rather than metastases. Renal cell carcinoma, hepatocellular carcinoma, adrenal carcinoma, and sarcomas are known to embolize to the lungs.

The CT scan is from a 76-year-old female who had a primary chondrosarcoma of the humerus which had spread to her right ventricle, and in this image the calcified mass (arrow) is seen within the right ventricle. There are also calcifications (arrowhead) within the right lung, as well as calcifications on the pleura. Courtesy of: Ashley Davidoff, M.D.
Other Emboli to the Lungs
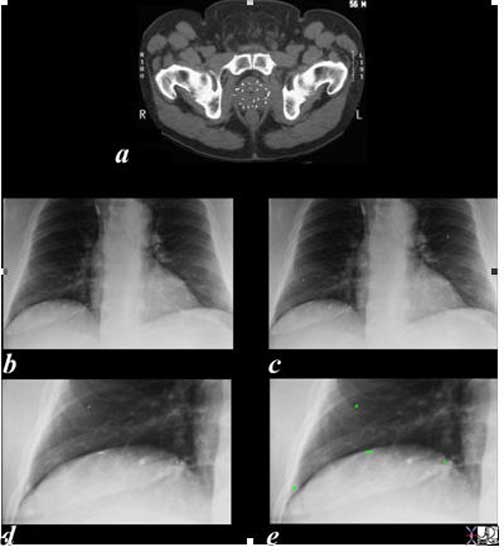
In this series of images of the patient described above the small radiation seeds are seen within the prostate parenchyma in (a), and are overlaid in green on the magnified views of the chest x-ray in (e) and (g). Presumably, they entered the veins of the periprostatic plexus (Batson’s plexus) and embolized to the lungs. They have no clinical significance.
Ashley Davidoff, M.D.
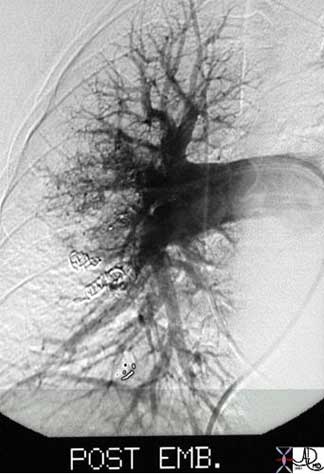
This angiogram is from a 54-year-old female with cirrhosis and hepatopulmonary syndrome characterized by a large arteriovenous malformation in the right lung. Multiple embolization coils (spring like structures) were deployed into the right lower lobe pulmonary artery in an attempt to occlude the feeding arteries and prevent the right to left shunting.
Images courtesy of: Ashley Davidoff, M.D.
Red Flags
As noted earlier, the diagnosis of a pulmonary embolism may be elusive at times. However, there are certain red flags that may hint to a diagnosis. First, an unexplained syncopal event may, in fact, be a result of a massive pulmonary embolism. Next, any patient with unexplained sudden onset, shortness of breath and rapid breathing should be considered for pulmonary embolism. Also, patients with any inherited genetic thrombophilia, such as, factor V Leiden, prothrombin gene mutation G20210A, and proteins C and S deficiency should be considered at higher risk for embolism if presenting with a consistent clinical picture.
Conclusion
Thrombotic pulmonary embolic disease is a dangerous and often silent killer. The clinical presentation ranges from a patient with no symptoms, to a patient who presents with acute cardiorespiratory arrest. High clinical suspicion must be maintained on all patients with unexplained cardiorespiratory symptoms, but especially those patients with a predisposition, such as the patient on oral contraception, oncology patients, and immobilized patients, for example. Clinical algorithms such as the modified Wells criteria have been very helpful and CT pulmonary angiography has become the mainstay of imaging.

