Clinical Strategies – Anatomy & Physiology of Pain

by Dr. Ashley Davidoff
Definition
Pain is a common symptom defined by the International Association for the Study of Pain as ?an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.? This statement characterizes the evolved nature of pain as a warning system and feedback mechanism that influences how we adapt to our environment. However, pain at its core is suffering and its persistence can be vexing to those who help the sick and often at a burdensome cost to society.
The causes of pain are innumerable and exist within the full spectrum of human diseases. A myriad of processes can occur in response to tissue injury leading to either irritation of a somatic nerve or distension and pressure on a visceral sensory nerve.
The diagnosis of pain is often problematic due to its connection to both benign and life-threatening conditions. Contemporary medical imaging can facilitate the diagnosis of pain by identifying a structural disorder early in the disease process. However, the degree of derangement may not always correlate to the severity of the suffering. It is, therefore, paramount for clinicians to use thoughtful questioning and a thorough physical examination to interpret pain within the context of other symptoms and findings.
Unlike most medical entities, pain management has not evolved to the point where objective scientific approaches are more reliable than subjective patient feedback. Pain is a valuable diagnostic tool and marker of treatment efficacy on one hand, and a labyrinth of intangibles, resistant to multiple therapies on the other. Effective pain management therefore, requires an understanding of its foundation and an artful approach to its subjective nature.
Although treatment options for pain can range from surgery to chronic palliation, it is important to remember that pain is a frequent human experience that is most often self-limited and/or easily treated with analgesics. When pain goes beyond the benign, however, it can represent one of the more challenging scenarios in clinical medicine. Healthcare providers, therefore, must balance proven treatment strategies with creative approaches to the pain mechanisms that are less well-defined. To that end, identifying the characteristics of different pain types is an important starting point.

Courtesy of: Ashley Davidoff, M.D.
Background to Entity
Clinically, pain is but one consideration in a constellation of symptoms and physical findings. As one of the most frequent patient complaints, pain is sometimes difficult to interpret as it can be indicative of both benign and life-threatening conditions. Furthermore, diagnosing pain can be a challenge when its location and pattern are elusive, widespread, recurring, or unremitting. Diseases like osteoarthritis and fibromyalgia, for example, are known to exhibit these pain patterns.
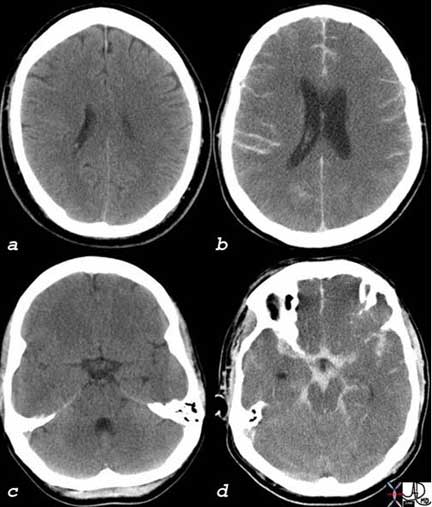
These two head CT’s (Computed Tomography) are both from two different patients who both presented with severe headaches. The first patient (a, c) had a normal study and the headache may have been caused by migraine, cluster, or tension headaches, for example. Although these headaches create significant morbidity, they are relatively benign in that they are treatable. The second patient (b, d) had a life-threatening subarachnoid hemorrhage due to a ruptured aneurysm characterized by the white hemorrhage in the sulci (b) and within the subarachnoid spaces surrounding the midbrain and other structures (d). It is not practical to perform a CT on every patient who presents with a headache, since it is a very common condition. On the other hand, one does not want to miss a life-threatening condition that is potentially treatable. Meticulous clinical acumen and prudent clinical judgment is necessary to direct management of patients with headaches and with patients who complain of pain.
The dura has the largest number of nociceptors of the meninges. In acute aneurysmal rupture, the severe pain is caused by stretching and distortion of the blood vessels that are accompanied by the nerves.
Courtesy of: Ashley Davidoff, M.D.
Classification
Pain is not a homogeneous experience and manifests in a variety of ways. Consequently, there are several ways to classify pain each with its own merit depending on the context of the case. For instance, pain may be described in terms of its location, its characteristics (colicky, sharp or dull), its duration or according to other factors. These and other classifications of pain will be explored in this tutorial.
Adaptive vs. Maladaptive Pain
As we start to understand and classify pain, it is important to view pain as an adaptive or maladaptive response. Adaptive pain is a physiological response that aims to protect the person by limiting activity in favor of healing. Acute and chronic pain can be adaptive. For instance, in the case of rheumatoid arthritis, there are acute cycles of inflammation which create an adaptive response which cause the patient to rest. There is also a subacute or chronic component of healing and repair which is a chronic adaptive pain. However, in the case of ongoing pain with or without obvious structural disorder or where the pain entity is counterproductive, non-protective, or a chronic encumbrance, it becomes a pathological entity and is non-adaptive. Psychocultural and psychosocial dynamics also become a large part of the pain syndrome and it, therefore, requires a different therapeutic approach.
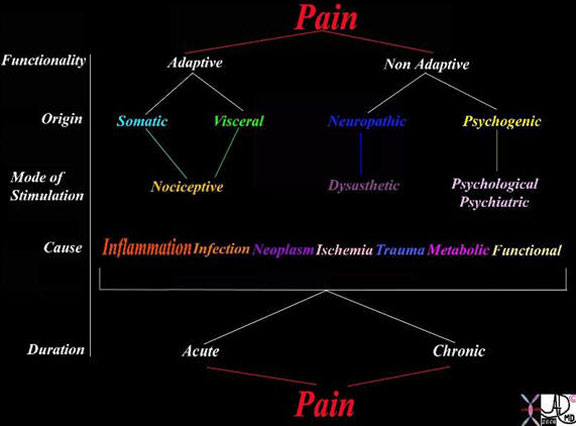
This table explores the variety of ways of classifying pain. The left hand column reveals the classification based on functionality, origin, mode of stimulation, pathological causes and relationship of pain to chronicity. As for functionality, it may be adaptive or non-adaptive. The pain may originate from somatic or visceral nociceptors or from damaged nerves in which case it is called neuropathic, or it may be psychogenic. The causes are usually via the inflammatory process but may result from any of the diseases listed.
Courtesy of: Ashley Davidoff, M.D.
Somatic Pain
Somatic pain is caused by direct injury of the skin, pleura, pericardium, peritoneum, muscles, bone, joint, or connective tissues. This type of pain is expressed in a variety of ways depending on the depth and location of the injury. The distribution of the somatic sensorium determines our ability to characterize and locate somatic pain.
From a functional point of view, sensitivity to somatic pain is a protective response and is mostly acute and immediate.
Somatic pain stimuli are mediated by nociceptors that reside in somatic tissue. Nociceptors are sensory neurons that are sensitive to painful or noxious stimulation. Indeed, these pain receptors are at their highest concentration in the skin and somatic linings of the body. Pain that is superficial or in an area of abundant nociceptors, therefore, is described as well-localized, sharp, burning or prickly; whereas, pain in an area of sparse nociceptor distribution is usually described as vague, dull, or aching.
Diagnosis of somatic pain is best accomplished through a thorough patient history and clinical examination. Characteristics of the pain may be deduced by palpation of the affected area. For instance, peritonitis is sensitive to minimal palpation pressure on the abdomen and usually entails rebound tenderness, which is discomfort brought about by slow, steady, downward pressure followed by a rapid release. The accompanying pain, called pleuritic pain, is acute, immediate, and short-lasting, but sharp. Pleuritic pain is another example of somatic pain. Movement or rubbing of the parietal pleura, which occurs upon inspiration, aggravates this pain even further and may serve as diagnostic indicators of peritonitis.
Treatment of somatic pain is often as simple as removing the insult and/or applying a simple analgesic.
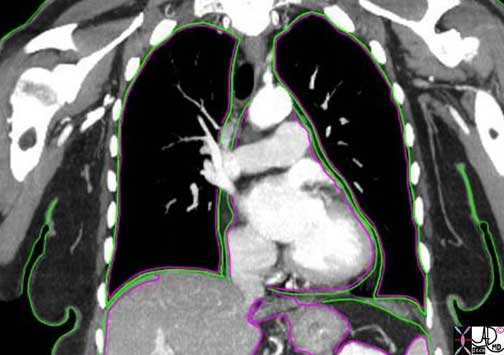
The example above is a coronal view of the chest as depicted by a CT scan. It demonstrates the somatic (green) and visceral (pink) linings of the chest. The skin as an outer layer contains a large variety and number of nociceptors, as does the outer parietal pleura (somatic -green) surrounding the lungs (black) and parietal pericardium (green) around the contrast-filled heart (white). The inner linings of the lung – visceral pleura (pink) and heart’s visceral pericardium (pink) are less endowed with nociceptors both in number and type, while the organs themselves have even less.
The somatic and visceral linings of the organs of the upper abdomen follow the same pattern and will be discussed below in more detail.
Courtesy of: Ashley Davidoff, M.D.
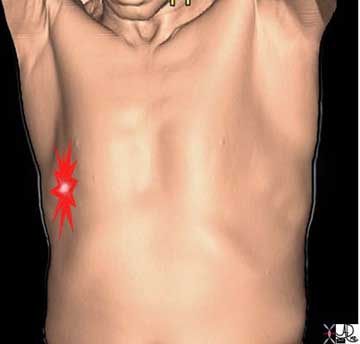
The pleuritic pain demonstrated on the right side of this patient is characterized by focal intense (bright red) and sharp pain that is aggravated by deep breaths and by coughing. The location corresponds to the disease and to the images described below.
Ashley Davidoff, M.D.

The pleuritic pain of pulmonary embolism, pneumonia or pleurisy is not distinct in itself, but the associated findings relating to the specific disease will help differentiate the variety of causes of pleuritic chest pain.
In this instance, a peripheral-based pneumonia abuts the pleural surface and parapneumonic pleural inflammation involves both the visceral pleura and the parietal pleura. When the lung moves with respiration, the rubbing of inflamed structures and particularly the pleura brings about the sharp focal pleuritic pain localized over the pneumonia.
Under some circumstances imaging may show enhancement of one of the somatic coverings.

This young male presented with right lower quadrant fever, peritonism and rebound tenderness and a thickened appendix and thickened peritoneum (red line) was noted on the CT scan. The diagnosis of acute appendicitis was apparent both clinically and radiologically and at surgery a hemorrhagic appendicitis was found.
Images courtesy of: Ashley Davidoff, M.D.
Visceral Pain
Visceral pain is caused by abnormal stretching of the visceral covering or the wall of an organ. Due to the lower concentration of nociceptors in the viscera compared to somatic tissue, visceral pain is subsequently less localizable.
Functionally, visceral pain is a protective response that serves as both a warning of disease and a means of limiting one?s activity such that further injury may be prevented.
Common causes of visceral pain include obstructions and ruptures of tubular systems in the body, which leads to distension and ischemia. In the case of the gastrointestinal tract and the genitourinary tract such distension results in colic or waves of severe pain.
As with somatic pain, visceral pain stimuli are mediated by nociceptors and thus proceed via the same neural pathway that proceeds through the spinothalamic tract to the thalamus and the inferior portion of the somatosensory cortex. However, these receptors are located deeper within the viscera themselves.
Clinically, visceral pain is often described as long-lasting, unrelenting, sudden in onset, colicky or cramping in nature, vague, ill-defined, or referred. For instance, a patient suffering from angina originating in the heart may complain of chest pain with radiating pain in the jaw or left arm. Each patient may have a different pain pattern for the same disorder which makes the evaluation difficult for the clinician. This phenomenon stems from embryological and neurological development.
Diagnosis of visceral pain is achieved through a thorough clinical history taking and examination and the prudent use of imaging techniques, which are especially useful in identifying obstructions and ruptures. Treatment of the obstructions and ruptures that cause visceral pain typically involves surgical or other invasive interventions.
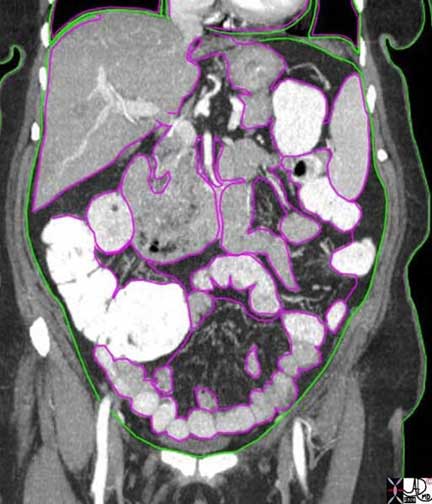
In this coronal reconstruction of a CT of the abdomen, the focus is on the pink visceral covering of the liver, spleen and gastrointestinal tract. Pain induced more by stretching of the organ rather than by a pinprick or cut can cause severe discomfort but it is poorly localized and often referred to other regions that have embryological implications.
Ashley Davidoff MD
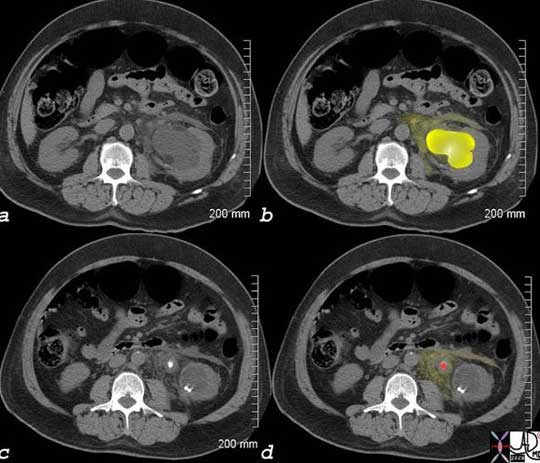
This patient presented with severe left back pain radiating to his testicle. The CT scan without contrast shows evidence of a stone causing obstruction of the left collecting system. In image (a) the normal right kidney is seen in relation to the hydronephrotic left kidney with a distended calyces and pelvis (bright yellow) in image (b) and a stone (red in image (d) in the middle of the ureter with some leakage of urine into the surrounding tissues. Ureteric pain is one of the most severe pains experienced by people and its prolonged course, colicky nature, and its pattern of radiation is typical of visceral pain.
Images courtesy of: Ashley Davidoff, M.D.
Neuropathic Pain
Injury to any of the peripheral or central nervous system components can be a source of neuropathic pain. This pain classification is unique in that the patient descriptors reflect a derangement in nerve conduction. Symptoms are described as either ?negative? or ?positive? due to respectively diminished or exaggerated sensation when stimulated. Usually the patient expresses neuropathic pain as a burning or an electrical sensation.
Typically, neuropathic pain reflects intrinsic, non-traumatic dysfunction of the peripheral nerves caused by damage to components of the sensory system including pain fibers, non-pain fibers, and 1st, 2nd, and 3rd order neurons. However, a variety of traumatic events and disease processes may lead to such damage, including chronic tissue injury, diabetic neuropathy, thermal injury, electrical burns, chronic repetitive injuries, toxic injuries (chemicals, neurotoxins, chemotherapy), autoimmune diseases, disorders of the thalamus and sensory cortex, and infections, such as herpes zoster and HIV.
Psychogenic Pain
Psychogenic pain is a syndrome characterized by seemingly real pain that exists without clinical findings. Instead, there is an underlying psychological disorder that is manifesting as pain. Clinically, the most common symptoms include headaches, muscle or back pain and abdominal pain. There are acute and chronic forms of this syndrome depending on timing of onset and response to treatment. The cause, intensity or duration of this pain, correlates to the degree of mental and/or emotional imbalance.
Mode of Stimulation Class: Nociceptive
Nociceptive pain is experienced when a noxious stimulus, such as a pinprick, burn, cut, hammer injury, colic, or cramps is translated into an uncomfortable experience. Some of these stimuli are experienced as a pricking sensation while others produce an ache or some combination of the two. The structures involved in nociceptive pain include the peripheral sensory first order neurons, the dorsal column of the spine, the spinothalamic tract, the thalamus, and the cortex. Somatic and visceral pain is primarily mediated through nociceptive pathways and may be described functionally by the electromechanical and electrochemical mechanisms that account for its perception. Typical treatment for this type of pain includes local, regional, and epidural anesthesia.
Dyaesthetic
Neuropathic pain is a maladaptive disorder of neuronal tissue characterized by aberrant and often unprovoked dysaesthetic impulses and does not involve nociceptors. There are subsequently many manifestations including hyperaesthesia (excessive sensitivity), hyperalgesia (excessive pain response), anesthesia (no sensitivity), paresthesia (pins and needles), allodynia (pain produced by a stimulus that would not usually cause pain), or dysaesthesia (aberrant unusual strong sensation).
Diagnosis of neuropathic pain involves a thorough exploration of the patient?s clinical background and history of unusual sensory symptoms. Unfortunately, due to its inferior healing capacity, neuropathic pain often becomes a chronic condition managed only by multiple tiers of therapy.
Psychiatric
Psychogenic pain is most often due to one of the following psychiatric disorders. These include depression, anxiety and bipolar disorder. This pain type is a maladaptive phenomenon of the brain where a biochemical imbalance is expressed as somatic or visceral pain.
The diagnosis of psychogenic pain is one of exclusion and is therefore often difficult to establish without exhausting expensive medical testing and imaging. It is important, however, for the clinician to understand that regardless of unsupported findings, psychogenic pain feels real to the patient and can cause significant disability. A multi-disciplinary approach with medical and mental health clinicians is therefore required to treat this syndrome and its underlying causes.
Causes – Pathological Classification
Inflammation
The pathological response to any injury in the human body is inflammation, but not always. This is an immune mediated storm initiated by increased vascular perfusion and permeability to the area of insult. Next, a fusion of cellular migration and degranulation leads to a release of peptides that stimulate among other things, specialized cellular pain receptors known as proteinases. The subsequent signals generated by inflammation cause symptoms that include burning, aching and pressure. Most causes of tissue injury invoke an inflammatory response. The mechanisms through which patients experience pain differ according to the injury process and tissue response, thus affecting its expression. Below is a summary of classical disease processes, each with a unique mechanism and subsequent pain signal.
Infection
Infection most often manifests as inflammation. However, the pattern and subsequent pain are different. Infection by nature delays healing. Pain complaints will therefore be more prolonged and progressive. Subsequently, inflammatory pain from infection will endure, deepen or spread until treatment is initiated.
Neoplasm
Pain in the setting of neoplasm is most often due to the mechanical effect of a tumor on the affected organ or adjacent tissue. Visceral or neuropathic pain will usually result, depending on the affected organ or area. In cases of metastases, bone involvement may become manifest as somatic (throbbing or aching) pain that can override the original neoplastic pain. Cancer therapy may also cause pain due to tissue burns from radiation or localized pain at the sight of a chemotherapy injection.
Circulatory – Ischemia
Insufficient perfusion of tissue leads to hypoxia, which is usually manifested as pain. The discomfort may be somatic as in the classic muscular ache of claudication or visceral as described by waxing and waning abdominal pain of ischemic bowel syndrome. The pattern of provoking or relieving factors are helpful descriptors in characterizing the supply and demand mismatch of compromised blood flow.
Trauma
The initiation of a somatic and/or visceral pain stimulus from direct tissue injury constitutes traumatic pain. The usual etiology is an external force, either blunt or penetrating, that is nociceptor mediated. Given the heterogeneity of nociceptor distribution, pain of traumatic origin varies widely according to the degree of injury incurred and the bodily location. Extraneous factors like age and previous pain experiences may influence a person’s reaction to the pain.
Like any other nociceptive pain, trauma can invoke local and systemic physiological and behavioral responses. At the cellular level, enzymatic and hormonal release will often cause inflammation. Systemically, respiration and heart rate can accelerate. Behaviorally, the pain threshold may or may not be reached resulting either in minimal reaction or complete withdrawal or immobilization of the body part to spare from further injury. When traumatic pain is severe enough, it may bestow long lasting, psychological consequences including Post Traumatic Stress Disorder (PTSD).
Muscular
Another pain pathway in the body arises from gamma sensors located in the muscle spindle apparatus of skeletal muscle. These receptors may be activated at rest or with motor function depending on the source. The most common form of muscular pain occurs after strenuous activity when muscle spindles have become relatively overused through stretching and contracting. The resultant achy pain associated with movement has often been controversially described as secondary to lactic acid and oxygen debt to the muscle fibers. One confounding factor, however, is that neuropathic pain is also transmitted in the same apparatus but at rest. Patients with spinal cord injuries may suffer from muscular pain as part of a syndrome called “Central Pain”.
Phantom Pain
The perception of pain following removal of a limb or organ constitutes the poorly understood disorder of phantom pain. The pain is physical and is thought to originate from neurophysiological adaptations in the nociceptor-mediated centers of the brain. However, attention to the treatment of the emotional sequelae that follow amputation or organ removal has been proven effective in mitigating phantom pain.
Classification of Pain Based on Chronicity
The relationship between a healed injury and either the relief or persistence of pain determines whether the experience is acute or chronic.
Acute Pain
Acute pain is a vital response to a noxious injury and is rapid in onset, can be severe, but is relatively short lasting, resolving as the injury heals or is treated. Its tenure depends on the healing rate of the injured tissue and can range from a fleeting moment to weeks or months of gradual relief usually less than 3 months. The usual healing time for tissues is really considered 4-6 weeks.
It is usually caused by mechanical or inflammatory disorders such as trauma, obstructions, ruptures, fractures, tears, strains, pulls, infections or acute inflammations.
Structurally, it relates to soft tissue damage.
Functionally, it is a sensory and protective phenomenon.
The diagnosis is usually resolved by a combination of careful history, sometimes requiring an imaging study for further documentation. In the hyperacute situation, autonomic responses such as tachycardia and hypertension are often present.
Treatment depends on the cause and may require surgical intervention.
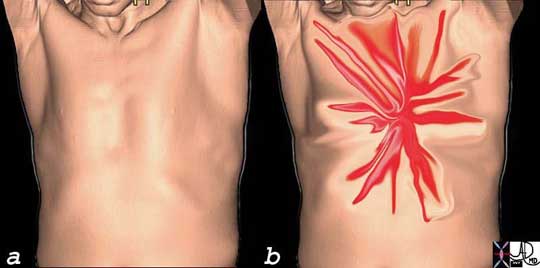
This image is intended to reflect the severe (bright red) lancinating, sharp, thunderbolt-like character (spiculated shape) of the chest pain that the patient with acute dissection may experience. One moment the patient is healthy without symptoms (a) and the next, there is catastrophic pain that reaches maximum intensity at the time of onset (b). This is one of the pain syndromes in medicine one fears. It represents one of the most severe forms of chest pain one can experience.
Courtesy of: Ashley Davidoff, M.D.

This X-ray is painful to look at. It shows an acute fracture through the fifth metacarpal and is associated with soft tissue injury. Periosteal disruption and injury accounts for the somatic pain experienced.
Images courtesy of: Ashley Davidoff, M.D.

This plain X-ray shows an acute fracture similar to that shown above, except it is a right 5th metacarpal fracture. It is called a boxer’s fracture since it is usually caused by a male youth hitting a wall in frustration with a closed fist. The bone most vulnerable in this situation is the distal 5th metatarsal.
The acute fracture associated with acute pain is shown in (a) and (b), while a healing fracture which still has minimal pain and discomfort is shown with bridging osteoid in (c) and (d). The pain at this stage is still considered acute since the tissues are still healing.
Images courtesy of: Ashley Davidoff, M.D.
Chronic Pain
Chronic pain is that which lasts more than three months and/or when pain dwells beyond a realistic healing time. Despite resolution of the injury, there is lingering activation of pain fibers perceived by the patient.
Unlike acute pain which is a survival mechanism, chronic pain is considered a disease state.
The cause of the pain may be identified but sometimes it is not. Rheumatoid arthritis, for example, is an autoimmune disorder that causes ongoing inflammation and is an identifiable structural disorder where the chronicity of the disease and the pain is easily understood.
The disorder may have a structural basis in which case diagnosis and treatment is easily accomplished. When and if there is little evidence for a structural basis and the syndrome has a bias to psychosocial and cultural origins, then the diagnosis and treatment is more challenging.
Functionally, the disease entity can be incapacitating irrespective of its structural or psychosocial origin.
Clinical attention to the nature of the pain, duration, precipitating and relieving factors and careful clinical examination is key.
There are often disabling features beyond the sensory manifestations of chronic pain, which may aid in the diagnosis. These include motor dysfunction, muscle tension, depression, personality changes, weight gain, anorexia, social withdrawal, and sleep disorders, for example, which favor the psychosocial syndrome.
The diagnosis is frequently made clinically and in the case of true organic disease, appropriate blood tests and imaging enable a diagnosis.
Treatment depends on the diagnosis. When there is a structural disorder, then treatment is usually straightforward. When there is a psychosocial origin, treatment is more complex.
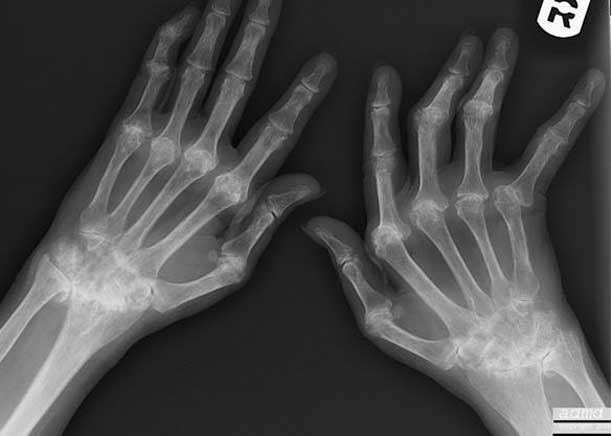
The plain film findings of rheumatoid arthritis are graphically depicted in both hands with erosive changes more prominent in the right (R) hand. There is overall ulnar deviation with irregularity and narrowing of the right middle interphalangeal joints on the right and bilateral involvement of the carpometacarpal junctions, and both proximal and distal carpal rows. Ongoing inflammation with cycles of destruction and repair lead to chronic pain.
Courtesy of: Ashley Davidoff, M.D.
Structural Basis of Pain
A pain impulse is initiated by sensory receptors called nociceptors which are located in almost all the tissues. A noxious stimulus, say from a hand touching a hot stove, is then transmitted by sensory nerves to the spinal cord where a direct spinal reflex causes immediate withdrawal from the source. Additionally, the stimulus is modified in the spinal cord by a variety of influences from other sources and is then transmitted via the midbrain and reticular activating system to the cortex. Finally, the stimulus reaches the brain’s somatosensory area where it is perceived and localized with additional extension to other areas of the cortex for the provision of a variety of protective reactions to the stimulus.
We will now expand the detail of the structural pathway described above.
The Sensory Receptors
Nociceptors are sensory neurons that are sensitive to painful or noxious stimulation. A nociceptor consists of free nerve endings which detect the stimulus, a long peripheral afferent fiber (peripheral process or primary afferent axon) that transmits the impulse to a ganglion cell, and a short fiber (central process) that takes the signal from the ganglion cell to the spinal cord.

The sensory receptors of the nociceptors are found in the tissues peripherally (top left) and are connected by a long fiber that transmits the impulse to the ganglion cell that lies in the dorsal ganglion in the neural canal alongside the spinal cord.
These specialized receptors vary in structure and number throughout the tissue and viscera of the body. There are external nociceptors that are situated in the skin and cornea with higher concentrations in the coverings of the body including the skin, pleura, pericardium, peritoneum and periosteum. Internal nociceptors are found in muscles, joints, around blood vessels and within the mucosa of some organs including the urinary bladder, genitourinary tract, and the gastrointestinal tract. There are nociceptors in varying concentrations in almost every organ in the body, but interestingly there are none in the brain substance itself.
There are two types of fibers that conduct painful stimuli; A delta fibers and C fibers. The speed of conduction relates to the diameter of the nerve and the presence of a myelinated sheath. A delta fibers are thicker and have a thin myelinated sheath while the C fibers are unmyelinated. Sharp or pricking pain is transmitted by the faster, medium-sized, myelinated A delta fibers which are responsible for the “immediate” pain experience, while the more prolonged, aching aspect of pain is transmitted by the smaller non-myelinated C fibers. Another type of fiber used in the modification of pain, but not sensitive to pain itself, is called the A beta fibers and they are heavily myelinated and thicker than A delta and C fibers. Hence, A beta fibers transmit their stimulus even faster.
A Delta Fibers
A delta fibers are sensory fibers that are structurally characterized by their intermediate size, thin myelin sheath, a single long peripheral fiber, a cell body located near the spinal cord in the dorsal root ganglion, and a short central fiber connecting the cell body to the dorsal root of the spinal cord.
Physiologically, the receptors are sensitive to pressure, (mechanoreceptors), have a high threshold to pain, – specifically sharp pain (high threshold nociceptors) and extreme hot and cold temperatures (>45°C or <5°C).
In the context of pain, A delta fibers are responsible for the immediate and first response to acute pain. They travel either to the spinal cord and cause a reflex muscle action to cause withdrawal from the immediate danger (hot plate example) and/or to the brain for the cognitive perception. Their lightly myelinated, medium-sized fibers allow conduction speeds of 5-30 m/s (about 40 miles per hour).
Clinically, the pain perceived from A delta fiber transmission can be variably localized depending on the concentration of receptors. Where nociceptors are high in concentration, localization can literally be pinpoint in accuracy. In areas of low concentration, the sensation may be felt over an area of 10-15cms. Either way, the localization of the pain is far better performed by the A delta fibers than that provided by the C fibers.

The A delta fibers consist of free nerve endings, are of intermediate size, are minimally myelinated (yellow sheath) and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
Courtesy of: Ashley Davidoff, M.D.
C Fibers
C fibers are polymodal sensory fibers that are structurally characterized by their small size, absence of myelin sheath, a single long dendrite, a cell body located near the spinal cord in the dorsal root ganglion, and a short axonal connection to the spinal cord.
From a functional standpoint, their receptors are sensitive to a variety of stimuli, including mechanical, chemical, and thermal stimuli and in the context of pain are the secondary responders to pain. Their unique non-myelinated fibers are slower conductors of the impulse which travels at about 2m/second (approximately 3mph).
Clinically, the pain perceived is throbbing or aching that immediately follows the hyperacute sharp pain, and in general is diffuse and sometimes even referred. However, since the C fiber activates the reticular activating system on its route to the brain, it may be associated with other visceral responses including increased heart and respiratory rates, nausea, vomiting, loss of pallor and even fainting.

The C fibers are small in size, are non-myelinated and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
Courtesy of: Ashley Davidoff, M.D.
Detection of the Stimulus – Free Nerve Endings
The actual receptors of painful stimuli are branched free nerve endings on the most distal portion of the peripheral process of both the A delta and C fibers. There are a few varieties of receptors which are specific to the type of painful sensation they can sense. Thus, there are mechanical, thermal, chemical, and polymodal nociceptors.

The diagram shows sensory stimuli including sharp pressure, extreme heat and cold as well as chemical, stimulating the free nerve endings of the nociceptors that are linked to the myelinated A delta fiber, and unmyelinated C fiber. The myelinated fiber will conduct the impulse between 3 and 15 times faster than the non-myelinated fiber.
Courtesy of: Ashley Davidoff, M.D.
All classes of nociceptors are present in the skin and tissues and work together in forming the pain response. For example, one may initially experience a feeling of “sharp” pain when hitting one’s thumb with a hammer, preceded by a prolonged “aching.” The initial pain occurs when A delta fibers transmit information from mechanical and thermal nociceptors to the brain. C fibers transmitting polymodal nociceptors are responsible for the latent but more prolonged aching experience.
The Dorsal Root Ganglia
The dorsal root ganglion (or spinal ganglion) is a localized conglomerate of the cell bodies of sensory (afferent) nerves that are situated on the dorsal root of the peripheral nerves just before they enter the spinal cord. They can be found within the neural foramen of the bony vertebral column. The dorsal ganglion for the facial structures is called trigeminal ganglion and is located in the skull, while the dorsal ganglia for the rest of the body are located along the spine.

The dorsal root ganglion is a focal accumulation of the first order nerve cells of the sensory component of the peripheral nerve (orange). It is situated in the neural foramen of the vertebral body. The central process emanates from the ganglion cell and ends in the dorsal horn.
Courtesy of: Ashley Davidoff, M.D.
The propagation of a pain stimulus requires three orders of neurons. The first order is as described above and is the neuron that brings the stimulus from the periphery to the spinal cord. Second order sensory fibers cross to the contralateral side of the spinal cord and connect to the thalamus. Third order sensory neurons then connect the thalamus with the sensory cortex.
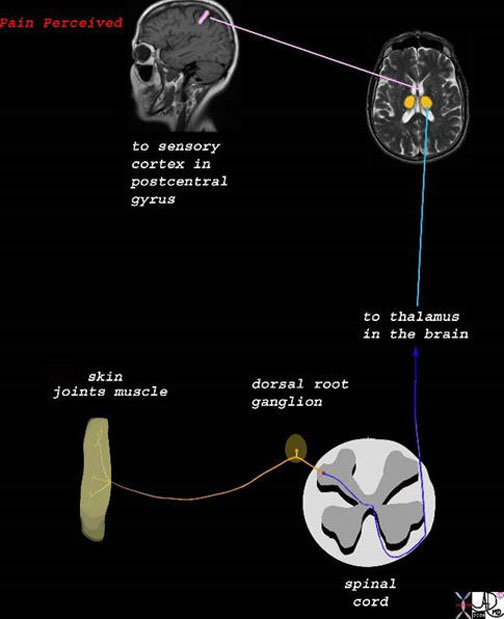
The stimulus is converted into an electrical impulse which is taken by a first order sensory nerve (orange) to the spinal cord (dorsal root ) which in turn transports the impulse via second order neuron (dark blue and light blue) to the thalamus,. The third order neuron (pink) transports the impulse to the somatosensory cortex.
Images courtesy of: Ashley Davidoff, M.D.
Role of the Spinal Cord
When the stimulus reaches the spinal cord, it travels up and down for a couple of segments in the white matter of the dorsolateral tract of Lissauer before it enters the gray matter of the dorsal horn. There it is processed and “read” and a variety of wave fronts are generated depending on peripheral, local, segmental and cortical influences. This process is called modulation and occurs in the substantia gelatinosa, marginal nucleus, or nucleus proprius. The impulse may, for example, be transmitted via a spinal reflex to cause an immediate protective withdrawal response. The signal can also induce a local autonomic response. The impulse then crosses to the other side of the spinal cord via a second order neuron and travels in the white matter tract called the spinothalamic tract via the medulla, pons, and midbrain to the thalamus.
The Gate Control Theory
The gate control theory (Wall and Melzack) is a theoretical mechanism that proposes that there is modification of the pain stimulus in the spinal cord by the interaction of relative stimuli from A delta, C, and A beta fibers. Once the nociceptors have been stimulated, an electrical impulse is generated and transmitted to the ipsilateral side of the spinal cord via nerve fibers from the dorsal ganglion to the dorsal horn. It may travel and connect with other neurons one or two levels up or down thus diffusing the accuracy of localization. The electrical impulse is read in the context of other sensory input including non-nociceptive information from the other sensory nerves such as the A beta fibers. Therefore, if only the A delta pain fibers are stimulated, the gate is opened and pain is perceived. If there is additional input from non-nociceptive fibers, then the gate is closed to a variable degree and pain can be reduced. For example, if a person suffers a bump on the head, the natural response is to vigorously rub the injured area. This induces and excites pressure receptors, thus transmitting the impulse rapidly over heavily myelinated and large A beta fibers. This causes a relative closing of the gate in the spinal cord, dampening of normal pain impulse conduction and thus, a modification of a painful sensation to one of pressure.
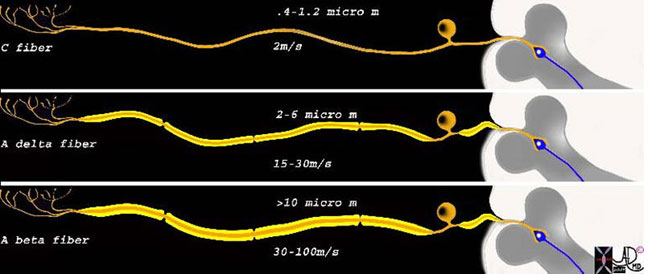
This diagram shows the three types of receptors and fibers that transmit impulses related directly and indirectly to pain. The upper fiber is called the C fiber and it is non-myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, measuring between 0.4 to 1.2 micrometers, and conducts the impulse at about 2 m/s. The second neuron is the A delta fiber and it responds to the pricking or sharp sensation that is first felt and reacted to. It is weakly myelinated and is about 2-6 micro meters thick, and conducts the stimulus with a velocity of between 15-30 meters per second. The last fiber is the A beta fiber and it is responsible for the pressure component which indirectly affects response to pain by affecting the gate mechanism of pain. It is greater than 10 microns thick due to heavier myelination and conducts impulses at 30-100 meters per second.
Courtesy of: Ashley Davidoff, M.D.
Spinothalamic TractAs described above, the second order neurons conduct the stimulus from the ipsilateral side of the stimulus to the contralateral white matter and the major pathway is then via the spinothalamic tract to the thalamus.
The spinothalamic tract is the major sensory ascending pathway of 2nd order neurons and serves as the major pathway for pain, temperature, itch and crude touch. Within its construct, the spinothalamic tract has three merging bands of specialized fibers that conduct characteristic pain impulses. The anterior spinothalamic tract carries pain signals initiated by touch while the lateral spinothalamic tract carries slow and fast fibers for pain and temperature sensations. The anterolateral spinothalamic pathway, located in the anterolateral white column pathway in the anterior half of the lateral funiculus conducts a variety of somatic pain signals.
In route to the thalamus, the second order neurons have to pass through the medulla, pons and midbrain. One of the systems that is activated by the C fibers in this path is the reticular activating system (RAS).

The pain fibers cross over the spinal cord via the second order neuron (blue) to the spinothalamic tract, the lateral spinothalamic tract and the anterior spinothalamic tract There are two parts to the anterolateral spinothalamic tract. The lateral spinothalamic tract (darker blue) carries the fibers for pain and temperature sensations and the anterior spinothalamic tract (light blue) carries sensation of simple touch.
orange = sensory nerve carrying stimuli from periphery
blue = anterolateral spinothalamic tract
dark blue = lateral spinothalamic tract
light blue = anterior spinothalamic tract
Courtesy of: Ashley Davidoff, M.D.
Reticular Activating System
The reticular activating system (also known as RAS, ascending reticular activating system) is a part of the brain considered to be the center of arousal and motivation. Structurally, it lies between the medulla oblongata and midbrain and is connected to the thalamus. In turn, all parts of the brain can be stimulated by the RAS including the cerebral cortex basal regions of the brain and the medulla. Functionally, the RAS indirectly relates to our state of consciousness and is involved with the control of the circadian rhythm, respiration, cardiac rhythms, and sexual function. In the instance of pain, the RAS is activated by the C fibers and hence, enables a painful stimulus to arouse us from sleep, create a sense of urgency, and can cause changes in heart rate or respiration rate.
Role of the Thalamus
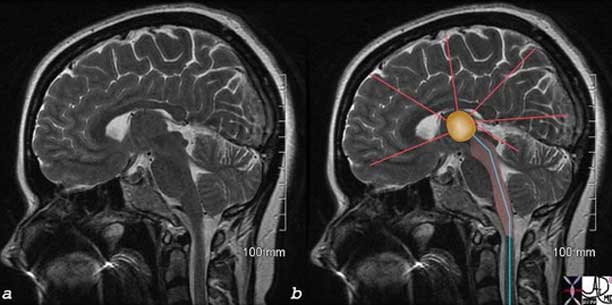
The image represents a coronal cut of the brain attained using T2-weighted MRI technique. It reveals a second order neuron (blue) traversing the medulla, pons, and midbrain, and in its path the C fiber component, is able to activate the RAS (pink). The stimuli reach the thalamus (orange) which not only activates the sensory cortex but also other parts of the cortex as well as shown by the red lines.
Courtesy of: Ashley Davidoff, M.D.
The thalamus is the gateway to the cerebral cortex. It is a paired organ and represents a major portion of the diencephalon.
Structurally, the thalamus has specific nuclei with diffuse projections to and from multiple regions of cerebral cortex.
The thalamus functions as a translator for the cerebral cortex. It processes sensory and motor information and mediates the autonomic nervous system regulating sleep and arousal. The thalamus also contains reciprocal connections to the cortex that are involved in consciousness. It may also play a role in vestibular function.
The thalamus translates pain signals of the 2nd order neurons and gives rise to the third order neurons that extend to the cortex. Awareness and localization of the pain is then achieved at the level of the cortex. The thalamus, however, is not merely a relay station for nociception but also plays a role in processing the stimulus.
Axons terminating in the lateral thalamus mediate discriminative aspects of pain (somatosensory cortex) including the originating body part. The fibers ending in the medial thalamus mediate the motivational and affective aspects relating, for example, to the emotion and memory of pain. These third order neurons travel to the prefrontal cortex, insular and cingulate gyrus, which contribute to the emotion and memorization of pain experiences.
Somatosensory Cortex in the Parietal Lobe
The somatosensory cortex is part of the somatosensory system and is characterized by its parietal location in the brain and by its ability to perceive and localize the pain.
Structurally, the cortex lies as the anterior most structure of the parietal lobe, positioned between the motor cortex of the frontal lobe and the central sulcus anteriorly, the post central sulcus posteriorly, and the lateral sulcus inferiorly.
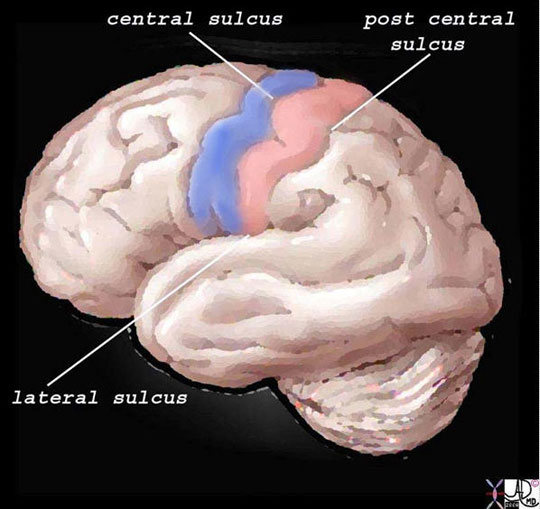
The somatosensory cortex is overlaid in light rose pink in the diagram above and represents the most anterior structure of the parietal lobe. It lies posterior to the motor cortex (blue) which is part of the frontal lobe, behind the central sulcus and in front of the post central sulcus. It serves to perceive, localize and evaluate intensity of the pain, as well as initiate the response to the pain.
Ashley Davidoff MD
The regions of the body have a specific location in the somatosensory cortex and depending on the number of nociceptors, will have a correlative sized distribution. Thus, for example, the lips, mouth, hands, feet, and genitalia will have a much larger representation in the somatosensory cortex than the limbs, trunk, and viscera. Subsequently, the various structures have a descending order of representation and consequently, a descending order of sensitivity.
If the somatosensory cortex is viewed in the coronal plane, the homunculus (literally “little man”) is draped over the sensory cortex with genitalia, and legs draped medially, thighs and trunk superiorly, and hands, head, mouth, lips, pharynx, tongue and viscera draped laterally. The size of organ representation is not only specific to pain fibers but also related to the sensory and motor system as a whole.
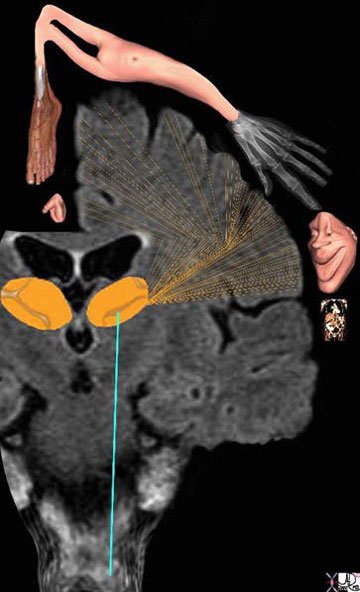
The diagram reflects the relative functional sensory space each body part occupies in the somatosensory cortex. Those structures with high density of sensory receptors are represented by a larger size, while those with a lesser concentration of sensory apparatus shown as being “smaller” in size. Hence, the mouth lips, hands feet and genitalia have a relatively large representation. The homunculus man (literally the “little man”) is the distorted figure drawn to reflect the concept of size of organ paralleling the size of the sensory innervation.
Images courtesy of: Ashley Davidoff, M.D.
The function of the somatosensory cortex is that of a higher processing center for touch, temperature, pain, and proprioception serving to amplify awareness of the sensations enabled by the thalamus. Sensation from the left side of the body is processed in the right somatosensory cortex and similarly those from the right side are processed on the left. The higher function of the somatosensory cortex allows us to localize the pain to a specific site, perceive the character and intensity of the stimulus, and sometimes helps identify the shape of the originating object.
The somatosensory cortex is not the final level of the somatosensory system since it also relays impulses to other cerebral areas of perception and reaction. Thus it sends signals via the white matter to other centers in the cortex to enable integration with visual and auditory input and with other higher cortical functions such as emotion and memory, for example. The full experience is then “seen” by the brain enabling the consequent reaction to be as discriminating and prudent as the nature and experience of the person allows. The difference between the reaction of an infant, child and an adult to the “shot at the doctors” speaks volumes about this latter function.
Head and Neck Pain
Pain syndromes of the head and neck vary according to the degree and area of injury but are generally poorly tolerated due to the anatomical confines of these regions. The head and neck anatomy is highly vascularized and well innervated with both somatic and cranial nerve fibers. Pain symptoms, therefore, are often accompanied by neurological findings. Causes of head and neck pain include vascular disorders, trauma, neoplasm, infection and treatment related syndromes after surgery or radiation therapy.
Headache is defined as pain in the head that is located above the eyes or the ears, behind the head (occipital), or in the back of the upper neck. There are many causes for headache but among the most common causes are migraine and tension headaches. The vast majority of headaches are benign and self-limiting and result in spontaneous resolution. Treatment is symptomatic aided with the use of appropriate analgesics. The most feared headache is the life-threatening event of a ruptured berry aneurysm. It is estimated that three out of four Americans had a headache at least once during the past year, and approximately forty-five million Americans suffer from chronic headaches, accounting for 80 million doctors’ office visits and more than 400 million dollars spent on over-the-counter pain relievers each year.
Neck pain is a common complaint seen in various medical settings including in primary care and the emergency department. It has many causes including dysfunction of the muscles and supporting structures such as bones and their joints (musculoskeletal dysfunction), infection, neurological disorders, pain from somewhere else in the body (referred pain, as in headaches or heart attack), and disorders of the various other structures in the neck including the esophagus and thyroid. Most neck pain is related to musculoskeletal dysfunction. In the U.S., almost 85% of all neck pain is thought to result from neck injuries (either acute or recurring) or from chronic stresses such as prolonged computer use. In the general population, the 1-year prevalence rate for neck and shoulder pain is 16-18% (Dreyer, 1998) and about two-thirds of people will experience neck pain at some time in their lives.

MRI of the brain showing distribution of somatic nociceptors (green) and visceral nociceptors (pink).
Courtesy of: Ashley Davidoff, M.D.
Chest Pain
Due to the prevalence of cardiovascular disease and its common manifestation of thoracic symptoms, chest pain receives priority status when described by the presenting patient. Chest pain is defined as the painful sensation that is classically felt in the front part of one’s body, between the neck and the upper abdomen. It can have many causes, that range from life-threatening conditions such as myocardial infarction and aortic dissection, to more benign conditions such as indigestion, heartburn, and gastroesophageal reflux, costochondritis, and muscular aches. We do, however, include in the module back pain that may reflect significant chest disease, such as pulmonary embolism, pleurisy, and aortic dissection, while also sometimes reflecting abdominal disease such cholecystitis and kidney stones.
Chest pain sometimes does not result in sufficient discomfort for the patient to seek medical attention, as unfortunately serious disease is not always associated with severe pain.
Diagnosis of the underlying causes often is the result of a careful clinical history and physical examination.Triage to subsequent diagnostic investigation usually includes a chest X-ray, EKG and cardiac enzymes.
Medical therapy for chest pain causes includes analgesics, antacids, anticoagulation and intravenous thrombolytics. Minimally invasive techniques for atherosclerotic disease may involve endovascular therapies like angioplasty, and stenting. Surgical interventions consist of coronary artery bypass and repair of dissections.

In the above diagram and through the text, we attempt to depict the nature of the pain diagrammatically. The more severe pains are shown in brighter red and the speculated shapes reflect sharper pain. Thus, the top left image is severe burning, retrosternal pain characteristic of gastroesophageal reflux disease (GERD) with esophagitis. The top middle image is a pressure-type pain that radiates to the neck, characteristic of angina and sometimes seen in esophageal spasm. The top right image is a more diffuse discomfort or pressure and is seen in angina and myocardial infarction. The bottom left image is sharp, lancinating, severe, almost devastating pain characteristic of acute aortic dissection. The focal sharp pain in the middle image, aggravated by deep inspiration, is characteristic of pleuritic pain and pericarditis, while the pain along a dermatome on bottom right is seen in herpes zoster (shingles).
Courtesy of: Ashley Davidoff, M.D.
Abdominal Pain
Excluding direct trauma, abdominal pain is often visceral in nature and therefore difficult to delineate. Visceral pain receptors are stimulated by distension, contraction, inflammation and ischemia. An understanding of these characteristics and a sound knowledge of abdominal anatomy can be helpful in demystifying abdominal pain.
The abdomen is a complex structure consisting of multiple spaces and compartments filled with a variety of heterogeneous organs and structures. The spaces and organs have been compartmentalized, divided and reclassified throughout the course of medical history according to the purposes of the specific group. For the clinician, dividing the abdomen into quadrants makes clinical sense. Right upper quadrant symptoms, for example, bring certain differential considerations and these are completely different from left lower quadrant symptoms. For the clinician who thinks embryologically, foregut, midgut and hindgut division makes intuitive sense. For the surgeon who has to decide about an invasive approach, division of the abdomen relates to upper or lower, and right midline or left. The approach mostly depends on the clinical presentation and can be divided into either a focused or global approach.
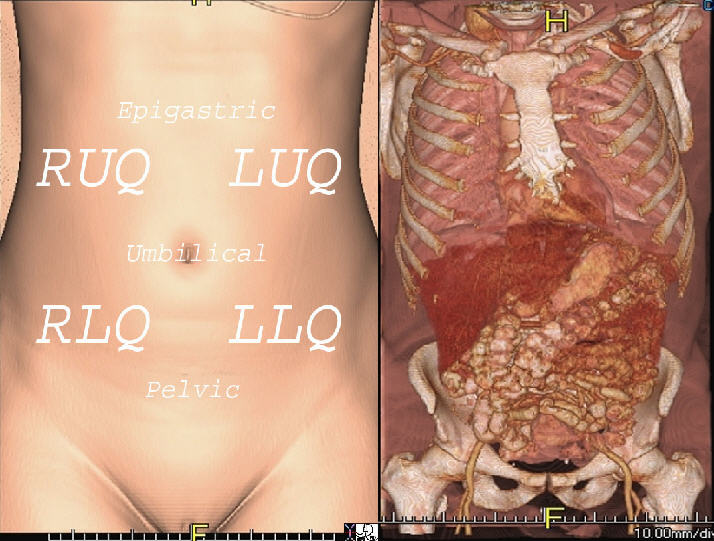
Shown are the normal anatomy from a CT, 3D Surface Rendering of the surface skin (left image) and internal structures (right image).
Courtesy of Ashley Davidoff, M.D.
Pelvic Pain
The pelvis is another highly vascularized area comprised mostly of the reproductive organs and a multitude of lumbar and sacral nerve plexus. Due to its anatomical proximity to the abdominal viscera, pelvic pain may often reflect an abdominal process.
Pelvic pain is an uncomfortable painful sensation felt in the pelvis. Pelvic pain is a common gynecological complaint, and can be broadly classified as cyclic, acute, or chronic. There are many causes for pelvic pain, with results that range from spontaneous resolution to some such as ruptured ectopic pregnancy that can be life-threatening. The diagnosis is often suspected clinically and imaging with ultrasound is the most commonly used modality in gynecological considerations. Treatment ranges from medical where pain control is the aim, to surgical where mechanical disorders such as obstructions and ruptures are the etiology.

Courtesy of: Ashley Davidoff, M.D.
Back Pain
Back pain is significant in its societal frequency and the impact on the workforce. There is a ninety percent lifetime incidence of back pain in the adult population and it ranks second only to the common cold for worker absenteeism. Although, classically back pain is a self-limited manifestation of muscle spasm in response to injury, disorders of the spinal structures and subsequent nerve roots can cause chronic, life-altering pain and disability.
Back pain is a disturbing and uncomfortable sensation felt in the lower or upper back. Low back pain may be caused by structural or functional disorders of the lumbar spine, intervertebral discs, nerve roots, spinal cord, muscles or ligaments. The pain may also originate from the bony pelvis or pelvic organs. Sometimes disorders in the upper abdomen can present with back pain such as gallbladder disease, kidney and pancreatic disease. Lastly, the skin of the back can also be the cause of back pain.
The clinical result ranges from fleeting pain to debilitating disease and sometimes to life-threatening disorders.
The diagnosis requires a careful clinical history that focuses on precipitating factors, duration, onset, character, situation, severity, aggravating, relieving, and associated disorders relating to the pain. Imaging may include plain X-ray films, CT scan, MRI or bone scan.
Treatment depends on the cause of the back pain and ranges from symptomatic relief with bed rest, analgesics and anti-inflammatory medication through physical therapy, to surgery when indicated.
Back pain has a wide distribution and includes structures of the thoracolumbar spine, chest and abdominal cavities.
Courtesy of: Ashley Davidoff, M.D
48390.83
Extremity Pain
The most common cause of extremity pain is injury to soft tissue, bone or cartilage that is either acute or repetitive. Pain radiation is common in the extremities due to alignment of sensory and motor nerves within the fascia. For example, repetitive stress injury to the forearm causing tendonitis in tennis players is often manifested as elbow pain thus invoking the nickname “tennis elbow”. Degenerative joint disease in the elderly is a frequent source of chronic pain and disability often with surgical joint replacement as the only remedy.
Physiology of Pain
The conceptual basis of any of the functional units in the body include an ability to receive, process and produce.
In this context, the sensory pain units receive a signal, process it and produce a result usually by a cognitive process and by transmission of a modified stimulus to another functional unit. In the context of pain there are three, broad, separate, functional transmission units that take the stimulus from the periphery, to the brain and then a host of processing units that run between each other before an integrated response is produced. The first, second and third order neuron have a relatively easy job. The chain of reactions that they cause in the processing units is complex and in many instances, not well understood, but the adaptive result in the end is designed to be protective and individualized, to a given patient based on their character and experience.
The first order neuron receives a stimulus from an internal or external environment of which it has some cognition based on the type of receptor stimulated and it then has to convert that stimulus into electrical activity. This process is called transduction. The fiber then transports the electrical activity along a selected neuron or group of neurons appropriate for the stimulus to a processing station, which for the neuron is a synapse. This second process is called transmission.
The synapse in the dorsal horn of the spinal cord acts as a processing station and is itself a functional unit. It receives stimuli from many sources, coordinates the stimuli from other sensory impulses such as the chemical milieu in the tissues and input from descending fibers, and transmits a modified stimulus to the second order neuron. The second order neuron receives an impulse and transmits the impulse in the spinal cord to the thalamus.
The thalamus receives the impulses, separates the stimuli into two discrete functional groups, provides some cognitive perception of the pain, and transports and directs the impulse both to the somatosensory cortex where cognitive localization is achieved and to the affective-motivational system where behavioral, emotional, and cortical functions are utilized.
The somatosensory cortex, limbic and autonomic systems, as well as the motor, cingulate, and prefrontal cortices coordinate effort and produce a response which may be in the form of a withdrawal, a cry or an autonomic response, but in addition, produce a fourth order in the process by sending modulation messages via a descending tract to the synapses between first and second order neurons.
Conclusion
Pain is no joke, and it has not been a joke for millions of years. It is noxious and obnoxious, but a necessary and sometimes a life-saving response to injury. Biology has complex ways of dealing with it, sometimes successfully and sometimes not successfully. Theoretically, for the adaptive type of pain it is a physiological response to an acute injurious agent and forces one to remove the body part from imminent danger. In the acute, subacute and chronic form it is also a physiological response because it forces one to rest the injured part allowing it to heal. On the other hand, when the pain is psychological or physical and evolves into a counterproductive result, then it is non-adaptive and pathological.
References
Bruckenthal P Pain Assessment Across the Life Span. American Journal of Nursing, 1984; 8, 981-985.
Fields, et al., Postherpetic Neuralgia: Irritable Nociceptors and Deafferentation. ??Neurobiology of Disease.?? 5, 209-227 (1998)
Hester N, Pain in children. Ann Rev Nurs Res. 1993; 11:105-142.
Hucho T., Levine J.D., Signaling Pathways in Sensitization: Toward a Nociceptor Cell Biology. ??Neuron.?? 55. (2007)
Kandel E.R., Schwartz, J.H., Jessell, T.M. (2000) Principles of Neural Science, 4th ed., pp.472-479. New York: McGraw-Hill. ISBN 0838580343
Levine D.N, (2007) Sherrington?s ?The Integrative action of the nervous system?: A centennial appraisal, ??Journal of the Neurological Sciences?? 253 pp1-6.
Merskey H, Bogduk N, Classification of Chronic Pain: Descriptions of chronic pain syndromes and definitions of pain terms/prepared by the Task Force on Taxonomy of the International Association for the Study of Pain. 2nd ed. Seattle, WA: IASP Press; 1994:222.
Purves, et al.. ??Neuroscience, 3rd Edition??, Sinauer Associates, Sunderland, MA.
Summers S, Evidence-based practice part 2: Reliability and validity of selected acute pain instruments. Journal of PeriAnesthesia Nursing, 2001; 16, 35-40.
Woolf C., Ma Q. Nociceptors?Noxious Stimulus Detectors. ??Neuron.?? (2007) Vol. 55. pp 353-364.
Web References
scientificamerican.com Genetic Underpinnings of Pain Sensitivity Revealed
ahfmr.ab.ca (Alberta Heritage Foundation for Medical Research) The mechanisms of pain and inflammation
medscape 5 articles and links
Warfield Carol A. Warfield, Zahid H. Bajwa Principles and Practice of Pain Medicine Published by McGraw-Hill Professional, 2003
Zang Xi-Chun Zhang, Andrew M. Strassman, Rami Burstein, and Dan Levy
Sensitization and Activation of Intracranial Meningeal Nociceptors by Mast Cell Mediators JPET 322:806-812, 2007
