Daniela Budiu, M.D. and Ashley Davidoff MD
Introduction
Chest pain is a common clinical presentation and its cause can range from a clinically benign musculoskeletal entity to a potentially life-threatening situation. In this module we explore the approach to the patient with chest pain in whom we suspect that the cause is cardiac in origin, based on initial clinical history and examination. The ?classical? patient may be a hypertensive, diabetic who presents to the emergency department with severe left-sided chest pain, pressure-like, for two hours. The pain could be associated with lightheadedness, palpitations and shortness of breath. Radiation to the jaw and left arm is another classical feature. Although angina may respond to nitroglycerin, the pain of acute myocardial infarction is not relieved.
Terminology
It is important to start by understanding some of the terminology. Acute coronary syndrome is a term that describes this clinical scenario and confirmatory diagnosis requires a 12 lead EKG, blood tests and close attention to vital signs. It is also important to distinguish between unstable angina and myocardial infarction. Two basic EKG forms are recognized:
- non-ST segment elevation myocardial infarction (NSTEMI)
- ST segment myocardial infarction (STEMI)
Acute Coronary Syndrome
Acute coronary syndrome (ACS) is the term used to describe any one of the three following entities:
- unstable angina
- non-ST elevation myocardial infarction (non Q wave MI)
- ST elevation myocardial infarction (Q wave MI)
Myocardial ischemia is the pathophysiological substrate for all the entities above, encompassed by the term acute coronary syndrome. Myocardial ischemia is caused by an imbalance between myocardial oxygen demand and supply secondary to coronary stenosis, obstruction, thrombosis or spasm. The chest pain in an acute coronary syndrome is caused by ischemia.
Terminology: Unstable Angina
Unstable angina (UA) is the clinical presentation of acute myocardial ischemia characterized by new onset chest pain within one month, chest pain at rest, chest pain lasting more than 20 minutes and not improving with nitroglycerin, chest pain more severe, more frequent and lasting longer than previously, without evidence of myocardial necrosis (no elevation of cardiac enzymes) and with or without EKG changes of ischemia. Unstable angina is a clinical diagnosis.
Terminology: Myocardial Infarction
Myocardial infarction (MI) is a circulatory disorder that is characterized by the necrosis of myocardial tissue caused by myocardial ischemia, and clinically characterized by the presence of symptoms of acute myocardial ischemia (ischemic chest pain or chest pain equivalent as dyspnea, diaphoresis, lightheadedness, palpitations), associated with characteristic EKG changes and biochemical markers of myocardial necrosis as reflected by typical rise and fall of cardiac enzymes: troponin I or T, CK-MB. Pathological diagnosis of myocardial infarction requires evidence of myocyte cell death (i.e. necrosis of the myocardium) as a consequence of prolonged ischemia.¹
An alternate approach to classifying these entities is based on the EKG, depending on the presence of elevation of the ST segment.
Terminology: ST Elevation
Non ST Elevation:
- Unstable angina (UA)
- Myocardial infarction (NSTEMI)
The incidence of finding thrombus in the coronary arteries in these patients is between 35-70%.
ST elevation:
- ST elevation myocardial infarction (STEMI)
The incidence of finding thrombus in the coronary arteries in these patients is about 90%.
The obstruction in unstable angina NSTEMI is the result of one of four main pathophysiologic processes:
1. plaque rupture with superimposed non-occlusive thrombus (the most common cause),
2. dynamic obstruction such as coronary spasm,
3. progression of a mechanical obstruction ( advancing atherosclerosis or reocclusion after a percutaneous intervention)
4. secondary unstable angina – a condition with high oxygen demand and low supply (tachycardia, anemia) superimposed on a compromised coronary bed due to atherosclerosis. In NSTEMI the thrombus is called “white” since it is platelet rich.
In STEMI, the most common cause of obstruction is total occlusive thrombus with total compromise of downstream blood flow. In rare cases, STEMI can be caused by total occlusion of a coronary vessel by a piece of tumor or other embolic material (intracardiac thrombus), coronary spasm, cocaine abuse or congenital anomalies. In STEMI, the thrombus is called “red” thrombus and represents classical thrombus.
The approach to the STEMI, therefore, is somewhat more urgent since the chances of finding and treating total thrombotic occlusion in the coronary arteries is higher. However, the chances of finding thrombus in the coronary arteries in the unstable angina patient and NSTEMI are still high but the thrombus is not usually totally occlusive. Vigilance and focused and emergency attention to all the entities is prudent.
Principles
Chest pain is ?an unpleasant sensory and emotional experience associated with actual or potential tissue damage and mediated by specific nerve fibers to the brain where its conscious appreciation may be modified by various factors.? In the case of the heart, insufficient supply of oxygen to the myocardium is the underlying principle, but the exact mechanisms of how the pain is caused are not well understood. We will focus on the underlying anatomical and pathophysiological principles of blood flow.
At its most basic, the coronary artery is a tube that transports fluid.
Ashley Davidoff, M.D
The coronary artery is more complex than a simple tube for many reasons. Its course is not straight but is curved. Many branches of varying size, changing resistances, and branching pattern with varied angles of origin make the flow more complex. The muscular and elastic nature of its walls and the viscosity and complexity of blood also contribute to the complexity of its physiology, pathophysiology, and imaging.
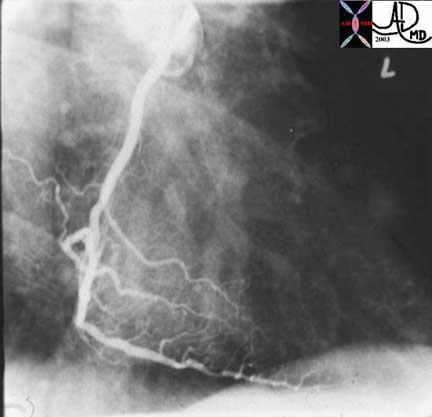
This is a normal right coronary angiogram in the right anterior oblique projection showing widely patent vessels. Note that it is not a simple straight tube but a branching structure with curving branches that come off at a variety of angles, that are subject to pulsatile flow.
Ashley Davidoff, M.D.
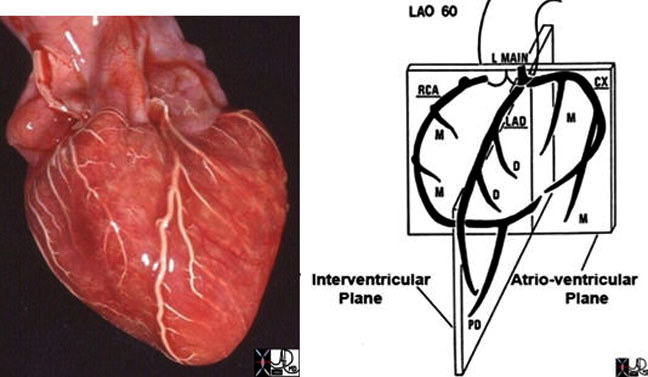
In the image above, the coronary arteries of the post mortem specimen have been injected with barium and thus look white. This anterior view shows the left anterior descending artery (LAD) coursing between the two ventricles to the apex of the heart. The diagonal branches (D) arise at acute angles and course over the left ventricle. The marginal branches (M) from the right coronary artery (RCA) are seen overlying the right ventricle, and the marginal branches (M) from the left coronary artery are seen overlying the left ventricle.The drawing on the right is provided to give a perspective of where these vessels lie.
Courtesy of: Ashley Davidoff, M.D.
Applied Anatomy
Blood supply to the heart is provided by three coronary arteries:
- Right Coronary Artery (RCA)
- Left Anterior Descending (LAD)
- Left Circumflex (CX)
Applied Anatomy: LAD and Circumflex

Copyright 2007 All Rights Reserved.

Copyright 2007 All Rights Reserved.
Applied Anatomy: Right Coronary Artery
The acute marginal branches (A1, A2, A3) originate from the proximal segment of the right coronary artery (RCA) and supply the right ventricular wall, which explains why an inferior wall MI (where the culprit vessel is RCA) can be associated with right ventricular infarction. In 60% of patients, the RCA also provides blood supply to the sinus node and this fact explains why sinus bradycardia can be seen in patients with acute inferior MI (RCA infarct). The posterior descending artery (PDA) is the biggest bifurcation of the RCA, running in the posterior interventricular groove and it supplies the posterior aspect of the interventricular septum. Atrioventricular nodal branch originates from the distal RCA after the PDA take-off and supplies the atrioventricular node explaining the presence of different AV node conduction abnormalities associated with inferior MI.
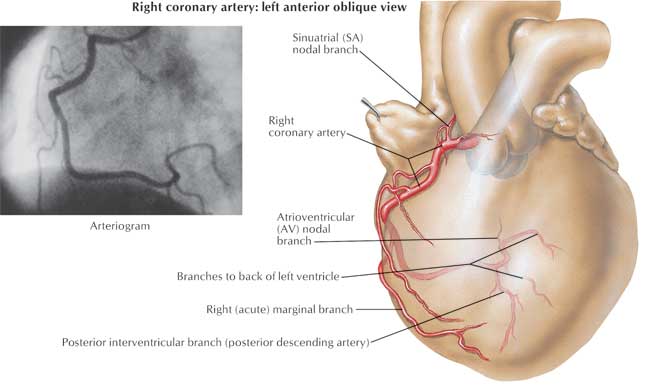
Copyright 2007 All Rights Reserved.
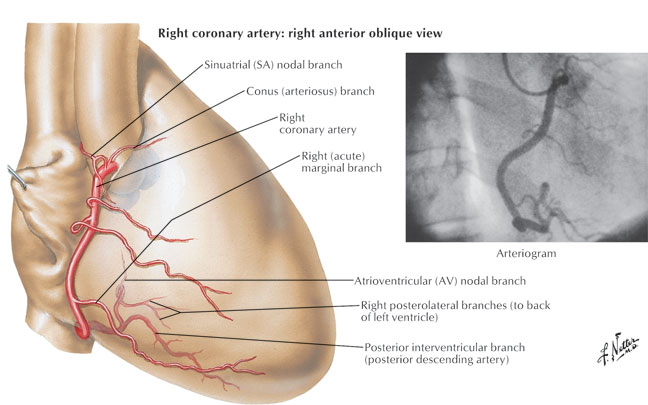
Copyright 2007 All Rights Reserved.
Pathophysiology of Acute Coronary Syndrome
Acute coronary syndrome (ACS) represents the progression of stable coronary artery disease to unstable disease. At tissue level, atherosclerosis of the coronary arteries is the predisposing factor to an acute coronary event. Fissure or rupture of vulnerable atherosclerotic plaque is the central event leading to an acute coronary syndrome.
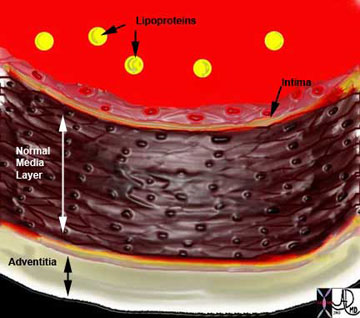
Ashley Davidoff MD
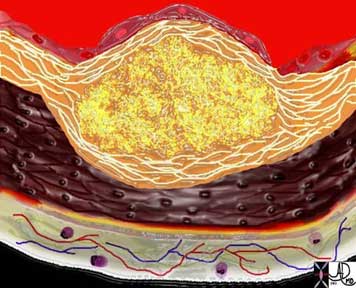
The diagram shows the atherosclerotic lesion in the subepithelial layer of the intima which is bulging both toward the media and toward the lumen. There is a central core of fat and necrotic debris, surrounded by fibrous elements which give the plaque its hardness to the feel. The accumulation of fibrous tissue heralds an advanced atherosclerotic lesion.
Ashley Davidoff, M.D.

Courtesy: Henri Cuenod, M.D.
Pathophysiology of Acute Coronary Syndrome: Atherosclerosis
Atherosclerotic plaque is seen radiologically in two forms. Fibrous plaque as seen in the angiogram below is characterized as a focal narrowing of the vessel while calcified plaque is better characterized by CT scanning and is exemplified below on the CT angiogram. The fat containing plaque is the most dangerous plaque but it is not routinely identified on current imaging techniques.
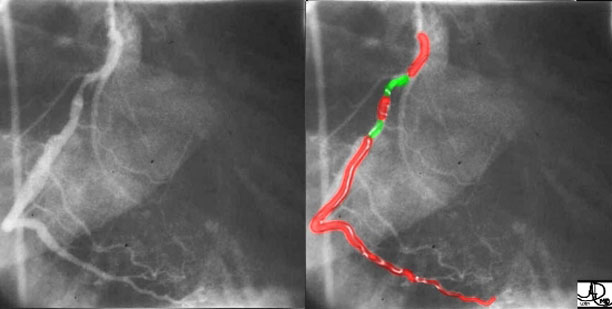
Coronary artery disease is the most common disorder that affects the heart mostly as a result of atherosclerosis which usually causes narrowing or obstruction of the vessel, but sometimes causes dilatation. In this instance, both narrowing and dilatation is noted. The injection of the right coronary artery is in this right anterior oblique (RAO) view with two narrowings outlined in green. The first narrowing is of moderate degree and the second is mild. A narrowing of 70% is considered hemodynamically significant.
Courtesy of: Ashley Davidoff, M.D.

Shown is a CT Angiogram (CTA) on a patient with atherosclerosis, demonstrating multiple calcifications (arrowheads) in the right coronary artery (white arrows).
Courtesy of: Ashley Davidoff, M.D.
Development of atherosclerosis reduces the lumen of the coronary vessel and limits the appropriate increase of myocardial perfusion during situations with high oxygen demand such as exercise, high emotional stress or even at rest if high degree of stenosis. When the stenosis of the vessel is equal to or greater than 75%, then anginal symptoms occur during those situations of high demand. When the vessel stenosis is greater than 80%, anginal symptoms may occur at rest. The degree of stenosis has a higher clinical relevance in pathophysiology of chronic angina. On the other hand, an acute coronary syndrome can result from an acute rupture of a plaque that doesn’t produce a flow limiting stenosis. Such lesions may not qualify as “significant” by arteriography based on the degree of stenosis only.
Pathophysiology of Acute Coronary Syndrome: Atherosclerosis Continued
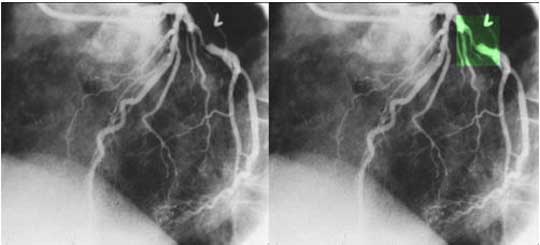
The coronary angiogram in the LAO projection shows a severe proximal stenosis (> 70%) of the circumflex with some post stenotic dilatation. The second image has a green overlay indicating the region of disease with the post stenotic dilatation. This lesion is considered hemodynamically significant.
Courtesy: Ashley Davidoff, M.D.
Acute coronary syndrome represents the progression of stable coronary artery disease to unstable disease. At tissue level, atherosclerosis of the coronary arteries is the predisposing factor to an acute coronary event. Fissure or rupture of vulnerable atherosclerotic plaque is the central event leading to an acute coronary syndrome. Plaque rupture can be triggered by physical exertion, increase in cardiac contractility, tachycardia, high blood pressure and possibly vasoconstriction. Rupture of the plaque leads to platelet activation and aggregation and activation of clotting cascade, resulting in the formation of a thrombus. If the thrombus causes total occlusion of the coronary artery, then ST segment elevation MI occurs. If the thrombus causes only incomplete obstruction of the coronary artery, then unstable angina or NSTEMI occurs.²
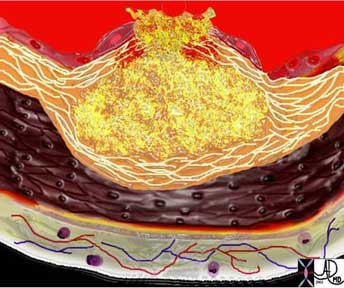
This diagram shows denudation of the endothelial layer with exposure and rupture of the contents of the atherosclerotic plaque in volcanic fashion into the lumen. This event is catastrophic and can result in acute thrombosis and may even be a fatal event.
Ashley Davidoff, M.D.

The histopathological specimen shows acute thrombosis of the coronary artery with red homogeneous thrombus (a) filling the lumen. B overlies the cholesterol plaque while c is fibrous plaque. D is the muscularis and e is the adventitia.
Courtesy of: Isabelle Joris, M.D.
Causes and Predisposing Factors
Non-modifiable risk factors for cardiovascular disease are increasing age, male sex, ethnicity and heredity (family history). Women’s risk of cardiovascular disease increases after menopause. On average, women manifest the disease 10 years later than men. However, at age 70, the risk of cardiovascular disease is equal in men and women. In women, concurrent diabetes confers a higher risk compared with men with diabetes.³
Family history plays an important role in the risk of developing cardiovascular disease later in life. Having a male relative who experienced MI before age 55 or a female relative with MI before age 65 places a person at a higher risk of cardiovascular disease.4
Also, different ethnic groups have different risks, with Mexican Americans, American Indians and Hawaiians being at higher risk for cardiovascular disease. The incidence of hypertension is higher in African Americans compared to Caucasians as well as their risk for cardiovascular disease.
Modifiable risk factors are diabetes, hypertension (HTN), chronic kidney disease, smoking, dyslipidemia, physical inactivity, and obesity. Lifestyle changes and drug therapies can modify risk factors leading to decreased morbidity and mortality in such patients.
Clinical Manifestation of Acute Coronary Syndromes
The patient’s history remains of critical value in the diagnosis of an acute coronary syndrome despite advances in biochemical markers, EKG and imaging modalities.
The classic symptom of an acute coronary syndrome is chest pain. Typically, pain is retrosternal or precordial and may radiate to the neck, jaw, shoulders, left arm or back (interscapular area). Pain is due to irritation of nerve endings in ischemic or damaged myocardium, but not necrotic yet. Pain resembles the chronic stable angina pain; however, in most patients is more severe in intensity, lasting for more than 30 minutes and more frequently for a number of hours, usually not relieved by nitroglycerin or by rest. Pain is being described as pressure, heaviness, tightness, squeezing or crushing and is triggered by physical activity, emotional stress or circadian rhythm. Usually pain responds to opiates, morphine in particular. Symptoms associated with the pain can be nausea, vomiting, palpitations, diaphoresis and subjective shortness of breath. Nausea and vomiting are more frequent with inferior location of myocardial infarction. When the pain is located in the epigastric area and is associated with nausea and vomiting, the clinical picture can be confounded with acute cholecystitis or peptic ulcer. Once again, the characteristics of the chest pain with myocardial infarction is very similar to that of unstable angina, but has been described as more severe in most cases.
Many patients experience less typical symptoms with myocardial infarction as a feeling of profound weakness and fatigue, dizziness, palpitations, cold perspiration without associated chest pain. Also, pain occurring in other locations than the chest, i.e. epigastric, numbness sensation of the chest or left arm, tingling sensation in the left wrist, hand or fingers, stabbing or burning pain, lightheadedness, or “indigestion” symptoms have been reported as initial presentation of myocardial infarction. These “atypical” symptoms of MI occur more frequently in women, elderly and in patients with diabetes mellitus, hypertension, heart failure and stroke.5, 6
In the elderly, frank syncope, as a sign of left ventricular failure secondary to massive myocardial infarction can be the initial presentation of STEMI.
Multiple studies have reported lack of typical chest pain in as many as 33% of patients experiencing an acute coronary syndrome.7
Differential Diagnosis
The chest pain as a result of an acute coronary syndrome should be differentiated by other causes of chest pain. Sometime the pain from MI may simulate the pain of acute pericarditis, therefore the big distinction is between the ischemic pain and pericardial or pleuritic chest pain as seen with acute pericarditis or pulmonary pathology respectively (i.e., pulmonary infarct, pulmonary emboli). A major clinical difference is that respiratory movements, taking a deep inhalation or coughing, aggravate both pleuritic and pericardial chest pain and the pain is reported as sharp, knife-like, which distinguishes from the deep, steady, dull pain of MI.
Aortic dissection also causes chest pain which is described by the patients as a “ripping” or “tearing” sensation radiating to the back and has maximal intensity shortly after onset. On physical examination, aortic dissection can cause absence of one of the major arterial pulse and blood pressure discrepancy between both arms.
Costochondritis can also mimic ischemic chest pain but the former pain is sharp and associated with marked localized tenderness.
Esophageal spasm also should be in the differential on MI.
Physical Exam
Patients with MI appear in distress, restless. They can have skin pallor and cold perspiration. Also patients experience difficulty breathing with severe left ventricular failure. If cardiogenic shock is present, the skin is cool and clammy with mottled appearance over the extremities and patients may present with confusion and disorientation secondary to cerebral hypoperfusion.
Vitals signs, including blood pressure (BP) in both arms, pulse and temperature should be checked. Most patients with STEMI develop fever in response to tissue necrosis in the first 24-48 hours after the onset of infarction.
The heart rate can vary from bradycardia – typically in inferior STEMI to sinus regular tachycardia or irregular tachycardia with frequent premature ventricular beats. Blood pressure is normal in the majority of uncomplicated MI; however, in previously normotensive patients in the first initial hours of the event the blood pressure may be elevated due to high adrenergic state. In patients with massive STEMI and cardiogenic shock, the arterial blood pressure falls acutely to level below 90 mm Hg. Hypotension and bradycardia can occur also secondary to increased vagal tone in inferior wall MI in the absence of left ventricular failure or cardiogenic shock.
Cardiac examination of patients with MI may be unremarkable or there may be clinical signs suggestive of ischemia or heart failure as a result of the acute ischemic event. On auscultation, the heart sounds may be decreased in intensity acutely. New systolic murmur can be heard as a result of transient/persistent mitral regurgitation caused by acute ischemic papillary muscle dysfunction or rupture. Paradoxical split second heart sound is present in extensive myocardial dysfunction and subsequent left bundle branch block (LBBB). Also, a third cardiac sound can be present, jugular vein distension or rales on lung auscultation if severe heart failure develops secondary to MI. Peripheral vessels should be examined for bruit or pulse deficit.
A careful physical examination may help to rule out aortic dissection, pneumothorax, pericarditis, cardiac tamponade or congestive heart failure.
Labs
The diagnosis of ACS/MI requires at least two of the following three criteria according to the World Health Organization:
- history consistent with ischemic type of chest pain
- changes on serial EKG tracing
- rise and fall in cardiac markers.8
EKG
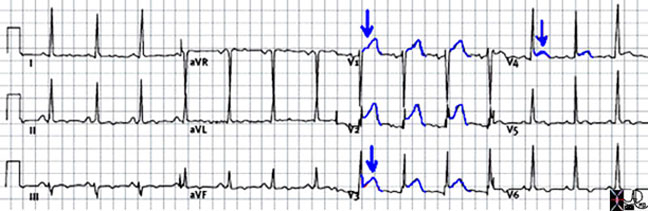
EKG is of crucial importance in the diagnosis of a MI. The EKG changes can help with the distinction between STEMI and NSTEMI, however is less useful to differentiate NSTEMI from unstable angina (UA). In UA, the EKG can show ST segment depression in the leads corresponding to the myocardial territory supplied by a coronary artery. In NSTEMI, there is typically ST depression, T wave inversion or nonspecific ST-T changes.
In STEMI, the first sign of an acute event could be acute elevation of T wave which is called “hyper acute T wave”, followed by ST segment elevation, then normalization of ST segment with development of T wave inversion and Q wave. When an ACS is being suspected by the history, the EKG should be repeated every couple of hours, as these changes are dynamical. Also, the EKG should be compared with an old one if available.
The changes of STEMI can be masked by the existence of LBBB. Any presumed new LBBB on EKG with a clinical presentation suggestive of MI should be managed as a STEMI.
EKG changes can be used to predict the location of MI.
| Location |
Leads |
Vessels |
| Anterior |
V2-V4 |
LAD |
| Anteroseptal |
V1-V4 |
LAD |
| Anterolateral |
V1-V6, I, aVL |
LAD |
| Diagonal | ||
| Inferior |
II, III, aVF |
RCA, LCx |
| Lateral |
I, aVL, V5-V6 |
LCx, Diagonal |
| Posterior |
tall R in V1-V3 |
RCA |
| Right Ventricle |
right V4 |
RCA |
| RCA=right coronary artery | ||
| LAD=left anterior descending |
Blood Tests – Cardiac Markers
The creatine kinase (CK), the creatine kinase MB (CK- MB) and troponins (I or T) are currently used as biochemical markers of cardiac damage. The cardiac enzymes should be checked on presentation every 8 hours until peak. The troponins are the gold standard for diagnosis of MI as they have higher sensitivity and specificity. Troponins can be detected in blood 4-6 hours post injury, peak at 24 hours and they remain elevated in serum for 7-10 days.
CK – MB is less sensitive and specific as it can be released from skeletal muscle as well as the tongue, diaphragm, intestine, uterus,and prostate. Both total CK and CK-MB are detectable in blood at 4-6 hours, peak at 24 hours and returns to normal in two to three days.
Myoglobin is used less in diagnosis on MI, but can be used when an urgent diagnosis is needed in the absence of EKG changes. Myoglobin appears in the serum 30-60 minutes after the onset of symptoms but falls quickly. It is less specific than troponin but has higher sensitivity, especially when used in conjunction with troponins.
Keep in mind that cardiac enzymes can be positive in a patient with chest pain caused by a massive pulmonary embolism which causes strain on the right ventricle releasing cardiac enzymes from right ventricular ischemia or infarct. Also, acute aortic dissection which involves the origin or coronary arteries can cause elevation of cardiac enzymes. Myopericarditis also is associated with elevation of cardiac enzymes. Usually the character of chest pain/EKG can help with the distinction between ischemia as a cause of elevation of cardiac enzymes and other pathologies (see Differential Diagnostic section), however, there are multiple situations when the clinical history is vague or unavailable so the diagnosis of MI is challenging and implies using different imaging modalities which are not routinely used (see Imaging section).
Cardiac enzymes should be checked on every patient with a history suggestive of acute coronary syndrome; however, in patients with ischemic chest pain and ST segment elevation on EKG or new left bundle branch block, the decision for reperfusion therapy should not be delayed waiting on cardiac enzyme results, as they may remain negative for the first few hours. Cardiac enzymes are more important in risk stratification and decision making in patients with NSTEMI and actually help in making the distinction between NSTEMI and UA.
Other Blood Tests
Lipid profile (serum total cholesterol, LDL cholesterol and HDL cholesterol) should be obtained at the time of initial presentation as the level may be falsely low after 24 hours since presentation. For patients admitted 24- 48 hours after the acute event, a lipid profile should be done 8 weeks after infarction has occurred.
Hematological Findings
Elevation of white blood cell count develops 2-4 hours after the onset of chest pain and reaches a peak 2-4 days after infarction and returns to normal in one week. The usual peak ranges from 12.000- 15.000/ml but can be as high as 20.000 in patients with large STEMI.
Markers of inflammation like erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) can be also elevated for a few days after infarction and they may remain elevated for few weeks. Other questions the physician would consider are:
- How quickly are these studies done?
- When do blood test results come back?
- Do you wait for these results before deciding on Cath?
- How long does it take for enzymes to come back?
quizme.gif |
| For patients admitted 24- 48 hours after the acute event, a lipid profile should be done _____ weeks after infarction has occurred. (Note: You will be given 2 tries to answer this question, then the answer will be provided.)
|
Imaging
Usually the diagnosis of acute coronary syndrome can easily be made if the clinical history, physical examination, EKG and serum markers (i.e. cardiac enzymes) are all suggestive of ischemia. Keep in mind that cardiac enzymes can be detected in the blood only 4-6 hours after the ischemic event so if the patient presents to ER immediately after the chest pain episode, the first set of cardiac enzymes may be negative even if the patient is experiencing a true MI. Further diagnostic imaging tests to rule out other possible causes of chest pain are usually done during the evaluation of a patient with a possible acute coronary syndrome when the history, physical exam and EKG tracing are not totally consistent with an ischemic event.
Imaging: Preliminary Studies
Portable chest x-ray (CXR) should be done routinely during evaluation of a patient with chest pain. It may help rule out some other causes of chest pain rather than ischemia; aortic dissection which would show up on CXR as a widened mediastinum, pneumothorax, or rib fractures. Also, CXR in patients with cardiogenic shock as a result of an acute MI will show acute pulmonary edema with preeminent pulmonary vascular markings and diffuse alveolar infiltrates. However, the radiographic appearance of pulmonary edema needs up to 12 hours of elevated left ventricular end diastolic pressure to develop and the pulmonary changes may persist on a CXR for two days after the ventricular filling pressures have normalized. The presence of congestion on CXR has been shown to confer a worse prognosis in both STEMI and NSTEMI.
Imaging: Non-Invasive Imaging
Transthoracic echocardiogram can be useful in diagnosing MI especially in patients with chest pain suggestive of ischemia but without characteristic EKG changes. Regional wall motion abnormalities in a patient with chest pain may support the diagnosis of myocardial ischemia. Echocardiography is also useful to rule out aortic dissection by identifying an intimal flap. Transesophageal echocardiogram may be superior to standard echo in identifying aortic dissection. Also, transthoracic echo can be used to evaluate right ventricular strain and dysfunction caused by massive pulmonary emboli. Color Doppler echocardiography is useful to identify the severity of mitral or tricuspid regurgitation secondary to MI and some potential mechanical complications of STEMI such as acute ventricular septal rupture, free wall rupture and cardiac tamponade. These complications usually occur 4-5 days after the acute MI.
Ruling out aortic dissection in the setting of an acute MI is of crucial importance as this is a major contraindication to fibrinolytic therapy. As mentioned above, it can cause chest pain with cardiac enzymes elevation and ST segment elevation due to dissection involving coronary arteries. Surgery will be the only treatment option in that particular circumstance.
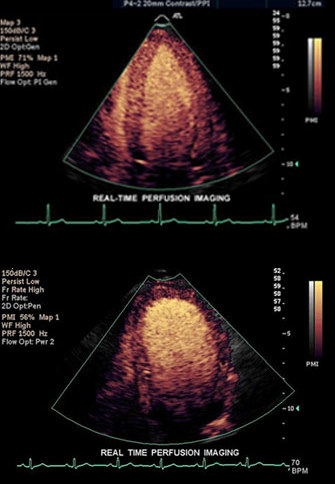
Abnormal LV (below)
This real time perfusion echo of the heart (left image) shows the left ventricle of a 4-chamber view and demonstrates homogeneous perfusion and normal conical shape of the left ventricle. The image on the right shows a dilated left ventricle revealing a heterogeneous and abnormal perfusion of the myocardium.
Courtesy of: Philips Medical Systems
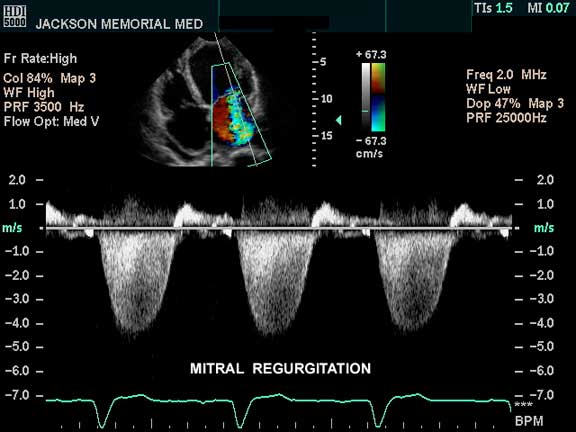
The 4-chamber view of the heart using pulse Doppler, as well as color flow Doppler, shows color changes in the left atrium which is abnormal and is pathognomonic of mitral regurgitation. If this finding is a new finding in a patient with AMI, it indicates severe structural damage. Papillary muscle rupture is one of the considerations in the setting of acute MI but it usually occurs about 8 days after the MI when the muscle is at its weakest.
Courtesy of: Philips Medical Systems
Imaging: CT Angiography
CT angiography (CTA) is not part of a routine diagnostic workup of MI. It can be used to rule out pulmonary embolism showing filling defect in the pulmonary vessels or dissection of the aorta in patients with chest pain and EKG changes non diagnostic of MI.
Imaging: Cardiac Catheterization
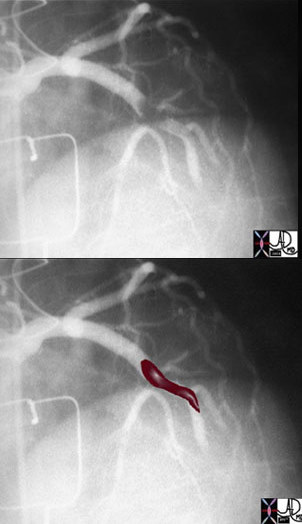
The above image of this cardiac catheterization shows a coned down left anterior oblique (LAO) projection of the LAD in a patient with acute thrombosis of the left anterior descending artery. In the lower image, the thrombus in the artery is overlaid in maroon.
Ashley Davidoff MD
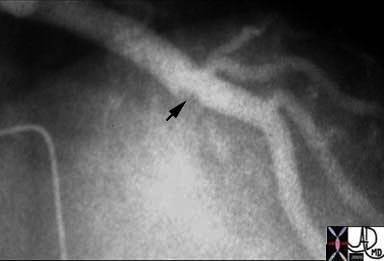
Following thrombolysis, minimal irregularity (black arrow) of the endothelium is seen. This may be residual thrombus or the remnants of a plaque rupture.
Ashley Davidoff, M.D.
Complications of STEMI
After a STEMI, patients can have mechanical complications (cardiogenic shock, free ventricular wall rupture, ventricular septal defect, and papillary muscle rupture), arrhythmia (atrial fibrillation, ventricular tachycardia, ventricular fibrillation, sinus bradycardia, and heart block), left ventricular aneurysm, left ventricular thrombus, and pericarditis. Mechanical complications usually occur 3-4 days after the acute event. They manifest as new onset hemodynamical instability (hypotension) and new murmurs on auscultation. Surgical repair is usually required.
Atrial fibrillation post MI occurs in 10-15 % of patients. It may require cardioversion if rapid ventricular response and hypotension occur. Otherwise, the ventricular rate should be controlled with beta blocker, calcium channel blocker or amiodarone.
Monomorphic ventricular tachycardia (VT) and ventricular fibrillation (VF) within 48 hours post MI does not carry bad prognosis. Antiarrhythmic (lidocaine, amiodarone) are used for monomorphic VT without hemodynamic compromise and cardioversion/defibrillation if ventricular fibrillation or pulseless VT. Electrolytes (potassium, magnesium) should be repleted (potassium >4 mEq/L, magnesium >2 mEq/L) and recurrent ischemia has to be ruled out. If VT/VF persists for more than three days after MI and there is no evidence of recurrent ischemia, then an implanted cardioverter defibrillator is advisable.
Sinus bradycardia and different blocks can occur post MI and therapy can vary from atropine to temporary or permanent pacing according to duration of block and presence of symptoms (hypotension, mental status change due to brain hypoperfusion, decreased urine output due to kidney hypoperfusion, etc).
Left ventricular thrombus can occur in 20-40 % of patients with large antero-apical MI and requires anticoagulation with coumadin for 3-6 months.
Ventricular aneurysm and pseudoaneurysm can also complicate a STEMI and sometimes require surgery. They can be easily identified on echocardiogram. Presence of persistent ST segment elevation on 12 lead EKG was classically thought to represent aneurysm formation but actually indicates a large infarct with regional wall motion abnormalities.
Pericarditis can occur after a MI and a rub can be heard on physical exam. Treatment consists of nonsteroidal anti-inflammatory, high dose aspirin and at the same time, a decrease in the anticoagulation medication (i.e. heparin, integrilin), as there is higher potential for bleeding.
Management
There are diagnostic and therapeutic priorities when evaluating patients with possible acute coronary syndrome.
The correct identification of the diagnosis of MI is of crucial importance and sometimes can be a major challenge. Furthermore, identification of patients with high risk features will dictate the therapeutic plan.
The ultimate therapeutic priority is restoring the coronary blood flow and minimizing the sequelae of ischemia. In addition to restoring the coronary blood flow, pain control and achieving hemodynamic stability is part of the immediate management in ER.
Triage begins with taking a good history and assessment of symptoms. Besides the classical symptoms of chest pain, suspicion also should be raised by “atypical” symptoms (see Clinical Manifestation section). Physical examination with recording of vitals, EKG tracing within 10 minutes from triage, CXR and draw of cardiac enzymes should be done. Patient should have continuous telemetry EKG monitoring of the cardiac rhythm, blood pressure and oxygen saturation monitoring by pulse oximetry. If the patient is hypotensive, hemodynamic resuscitation with intravenous fluid (normal saline as bolus) should be given. Also, the patient may benefit from vasopressor medication. If supraventicular tachycardia is present, metoprolol 5 mg IV every five minutes can be administered until heart rate is controlled provided the patient is not hypotensive. If ventricular tachycardia is present and the patient is hemodynamically stable, antiarrhythmic therapy with lidocaine, amiodarone is appropriate. If unstable ventricular tachycardia or ventricular fibrillation, electrical defibrillation or cardioversion is advised.
Risk factors should be also taken into account when evaluating a possible acute coronary syndrome as they help with the prognosis and with the decision where to admit the patient.
TIMI risk score is a useful clinical tool to stratify the patient’s risk of adverse outcome following a diagnosis of UA/NSTEMI. The seven risk factors are: age>65, more than three risk factors for CAD (see Risk Factors section), known past medical history of CAD, aspirin use, severe angina on presentation, ST segment deviation, and positive cardiac markers. Each of these seven factors gets one point, with a maximal score of seven. The higher TIMI risk on presentation, the higher risk of adverse outcome of UA/NSTEMI and more benefit from an aggressive therapeutic strategy. 9
The chance of finding a thrombus is not the element that influences therapy but rather the extent of myocardial damage secondary to the obstruction, and the percentage of myocardial mass that can be salvaged by an early intervention. The entire clinical picture (patient stability, risk factors as outlined above) are important considerations when management decisions are being made. In STEMI, irreversible necrosis of the myocardium will occur if blood flow is not restored in a timely fashion as the blood vessel is totally occluded by the thrombus. In NSTEMI, the decision to go to cath lab relies heavily on the TIMI score.
Management: Where the patient gets admitted to?
Patients with any of the following high risk criteria should be admitted to the coronary care unit or the step-down unit:
- age older than 75
- ongoing chest pain at rest for more than 20 minutes
- accelerating symptoms within last 48 hours
- signs of pump failure (hypotension, lung rales, pulmonary edema) associated with ST segment depression or elevation on EKG and positive enzymes should be admitted to the coronary care unit or the step-down unit.
Patient at intermediate risk with any of the following criteria:
- prior history of coronary artery disease (prior MI, prior CABG, prior aspirin use)
- history of cerebrovascular disease/peripheral vascular disease
- age more than 60, without ongoing chest pain
- T wave inversion on EKG and only slightly elevated troponin can be admitted to either the telemetry floor or step-down cardiac unit.
Similarly, there is a TIMI risk score for STEMI taking into account age, history of DM/HTN/angina, blood pressure< 100 mm Hg, HR> 100, heart failure on clinical exam (i.e. lung rales), weight <67 kg, anterior location of MI or new left bundle branch block and delay to therapy of more than four hours. Again, the higher the score, the higher the mortality would be from the acute STEMI.10
Management of UA/NSTEMI
The management of UA/NSTEMI consists of pain control/adjunctive therapy and restoring the coronary blood flow (antithrombotic therapy +/- angiography). Based on the TIMI risk score (see below), a conservative strategy versus an invasive strategy may be chosen. Patients with a TIMI risk score higher than three benefit from an early invasive strategy with percutaneous angiography.
If an invasive strategy is considered and angiography +/- angioplasty is being planned based on a higher TIMI risk score (>3) on initial evaluation, then the patient may be started on Glycoprotein IIb/IIIa inhibitors drip (i.e. Integrilin) in addition to aspirin and heparin before the angiography. Clopidogrel (i.e. Plavix) can be deferred in the invasive strategy for increased bleeding risk.
Conservative management (anti-thrombotic therapy) is usually chosen for low risk patients (TIMI risk score 0-2) and consists of aspirin (162-325 mg orally- first dose should be crushed/chewed to allow faster absorption through the oral mucosa), clopidogrel with 300 mg first loading dose, then 75 mg daily. Patient should be started on an unfractionated heparin drip or low molecular weight heparin. The patient will have a noninvasive stress test before he is discharged home. If the stress test is positive for ischemia, then the patient should undergo an angiography. If the patient develops recurrent chest pain during the hospital stay, then angiography will be reconsidered.
Pain control can be achieved by placing the patient on oxygen regardless of the level of oxygen saturation on pulse oxymetry, nitrates administered sublingual, oral, topical or intravenously. Adjunctive therapy consists of beta blocker (metoprolol 5 mg IV every 5 minutes provided heart rate is greater than 60, blood pressure is greater than 100, and there is no heart block or severe bronchospasm). Calcium channel blockers (i.e. Verapamil, Diltiazem) can be used to slow down the heart rate and for high blood pressure if the patient cannot tolerate beta blockers due to coexistent morbidities (severe asthma/COPD). Morphine is also used to relieve chest pain not responding to nitrates and also will help with relieving the signs of pulmonary congestion.
It is very important to monitor for symptoms and signs of bleeding while the patient is on the above antithrombotic medications, like external bleed which would be obvious versus internal bleed which would manifest as hypotension, tachycardia, drop in hemoglobin and hematocrit.
Management of STEMI
If the EKG changes are consistent with STEMI in the context of clinical history consistent with an acute coronary syndrome, then the reperfusion therapy is the core piece of management. Time is crucial in the management of STEMI and the outcome is better as the time to achieve successful reperfusion is shorter.
There are two methods to achieve restoration of the blood flow in STEMI depending on the level of the medical facility:
- Fibrinolysis (the process of dissolution of fibrin in blood clots).
- Percutaneous Coronary Intervention (PCI)
Fibrinolysis therapy is chosen when there is no catheterization lab available in the facility and the time to transfer the patient to another facility may take too long. Fibrinolytic therapy should be given within the first 30 minutes of arrival at the hospital (door to needle time <30 minutes) if the patient had symptoms of an acute MI for less than 12 hours with new LBBB or STEMI on EKG. The benefit of fibrinolytic therapy is less clear beyond the first 12 hours since the onset of symptoms.
There are absolute contraindications to fibrinolytic therapy (such as any prior intracranial bleeding, ischemic stroke or head trauma within 3-6 months, active bleeding, intracranial neoplasm, suspected aortic dissection) and relative contraindications (systolic blood pressure> 180, INR>2, recent internal bleed within 2-4 weeks, prolonged CPR> 10 minutes, trauma or major surgery in 2-4 weeks, pregnancy etc). The thrombolytic agents are Tenecteplase (TNK), Alteplase (TPA), and Streptokinase (SK). In addition to fibrinolytic therapy, patients should receive 162- 325 mg of aspirin (chewed or crushed) and heparin (low molecular weight heparin being better than unfractionated heparin in patients with age <75 and creatinine<2).
Primary percutaneous coronary intervention (PCI) should be done within 90 minutes of arrival at the ER (door to balloon time = 90 minutes). Primary PCI is superior to fibrinolysis; however, there are many hospitals without a cath lab available 24/7. PCI should be considered especially if cardiogenic shock, anterior MI or heart failure is secondary to STEMI. Patients in which primary PCI will be done should also be started on antithrombotic therapy, i.e. aspirin, unfractionated heparin drip and glycoprotein IIb/IIIa inhibitors drip.
In addition to fibrinolytic therapy, for the pain control in STEMI please refer to the management of UA/NSTEMI (i.e. oxygen, nitrate, morphine, beta blocker).
Management: TIMI Risk Score
TIMI risk score is a useful clinical tool to stratify the patient’s risk of adverse outcome following the diagnosis of UA/NSTEMI. The seven risk factors are:
- age >65
- more than three risk factors for CAD (see Risk Factors section)
- known past medical history of CAD
- aspirin use
- severe angina on presentation
- ST segment deviation
- positive cardiac markers
Each of these seven factors get one point, with a maximal score of seven. The higher TIMI risk on presentation, the higher risk of adverse outcome of UA/NSTEMI and more benefit from an aggressive therapeutic strategy. 9
Similarly, there is a TIMI risk score for STEMI taking into account: age, history of DM/HTN/angina, blood pressure< 100 mm Hg, HR> 100, heart failure on clinical exam (i.e. lung rales), weight <67 kg, anterior location of MI or new left bundle branch block and delay to therapy of more than four hours. Again, the higher the score, the higher the mortality would be from the acute STEMI.10
Red Flags
As mentioned previously, the diagnosis of an acute myocardial infarction can sometimes be very challenging even for the healthcare professionals so the patient?s awareness of red flags for an acute cardiac ischemic event leading to an emergency room visit can be potentially life saving. Presence of any of the following conditions/characteristics represents a red flag which should raise the patient or the medical staff suspicion for a MI.
- Personal risk factors (HTN, DM, hyperlipidemia, smoking etc)
- First episode of chest pain
- Chest pain at rest occurring in patient with history of exertional chest pain
- Chest pain not relieved by nitroglycerin
- Chest pain lasting longer than 20 minutes
- Shortness of breath (SOB), “indigestion symptoms” like nausea or vomiting, diaphoresis, left arm numbness (see Clinical Manifestations),
- EKG changes (ST elevation or depression)
- Positive cardiac markers of ischemia – those can be negative on first blood draw!!!
Patient Information
Experiencing a MI can be a very stressful event in a patient?s life, so during the initial ER presentation providing appropriate information about the diagnostic, therapeutic options, expected complications from both the acute event itself and from therapy is of crucial importance.
The initial focus of the patient presenting with a suspected MI is one of confirming the diagnosis using a combination of blood tests, EKG patterns, and possibly an angiogram. This is sometimes obvious immediately and sometimes takes a few hours.
Once the diagnosis of STEMI is made, patient and family should be aware of the two main therapeutic options available; intravenous fibrinolytic therapy or cardiac catheterization with local thrombolysis, angioplasty and vascular stenting (based on individual hospital level). Patients should be informed of major complications of each option, i.e. bleeding with fibrinolysis and groin hematoma, bleeding, and renal failure with cardiac catheterization. They should be informed that prognosis is dependent on the time to reperfusion; the sooner the better.
Patient and family should be aware that the first 48 hours after a massive heart attack can be critical, with most arrhythmic complications occurring during this period. Also, they should be informed that fatal mechanical complications can occur with ST segment elevation MI even a few days later after the acute event. The extent of the cardiac muscle damage will dictate the overall prognosis and this information can be obtained with an echocardiogram of the heart to assess the residual cardiac function after the MI.
Later on during the hospital course, the caregiver should inform the patient about the overall prognosis and impact on lifestyle. Secondary prevention strategy aims to help decrease the chance of a future event.
The time before discharge is an important opportunity to communicate risk factor modification. Patients should be informed about the importance of achieving optimal weight, daily exercise, appropriate diet, smoking cessation, blood pressure control, lipid control and hyperglycemia control for diabetic patients. The importance of medication compliance must be reinforced. Long term mortality improvement has been shown with antiplatelet therapy, aspirin, clopidogrel for prevention of thrombosis, beta blockers for hypertension and angina, statins for hyperlipidemia, and angiotensin converting enzyme inhibitors for heart failure and hypertension.
Before discharge from the hospital after a STEMI, every patient should receive appropriate counseling regarding the level of physical activity they can safely perform. Initial physical activity should consist of ambulation with multiple daily rest periods. Patients should avoid isometric exercise such as lifting and any activities that evoke symptoms. The activity level should be gradually resumed as convalescence progresses. Regarding resuming sexual activity, this should be done after successful completion of a modified stress test. Patients should receive prescriptions for nitroglycerine and be instructed as to how and when to use the medication and also detailed information on all prescription medications they should take. Patients should also be aware of all atypical symptoms of MI and have a low threshold to seek medical attention in the future. Cardiac rehabilitation programs for patients with STEMI are also available and gradual increase in the level of physical activity under direct supervision of qualified personnel is recommended.
Given the association between depression and STEMI, psychological counseling should be offered to these patients.
Routine office visits every 4-6 months are suggested for the first year after the index event and annual visits afterwards. With every visit, the physician should address five questions:
- Has the patient decreased the level of physical activity since the last visit?
- Did the patient experience any chest pain since the last visit and if yes, in which circumstance and what are the characteristics (duration, severity, level of activity to provoke it)?
- Is the patient compliant and tolerating therapy?
- Has the patient attempted to decrease the risk factors for cardiac disease?
- Any new comorbidity that may increase the risk of ischemic heart disease? The patient should be provided with the web address where he can find additional information about heart disease.
Conclusion
?Chest pain suspect MI? is an emergency and not a straightforward recipe algorithm. The diagnosis is not always clear, the potential life-threatening complications are real and anxiety provoking, and the treatment options are often time limited and based on quick and accurate diagnosis. All patients in this category need an urgent focus. To this end the first step is diagnosis, and although clinical presentation is highly suggestive, initial confirmation is based on EKG and blood tests. A first draw of the blood tests may be falsely negative and the EKG is also not always reliable. It is important to distinguish between STEMI and NSTEMI because the entities are approached differently.
The major difference pathologically between the two entities is that STEMI is usually caused by totally occlusive “red” thrombus while in the unstable angina group and NSTEMI the most common pathology is “white” platelet rich thrombus which is generally non occlusive.
The approach to the STEMI, therefore, is urgent catheterization to confirm the presence of occlusive thrombus and to institute thrombolysis. NSTEMI is also life-threatening but is usually managed conservatively with medication, although angiography usually is performed during the admission. Although the STEMI has an initial higher mortality due to a larger area of infarct and many potential complications, the one year survival is equal in both STEMI and NSTEMI. Patients with NSTEMI have a smaller size of infarct and decreased early mortality, but they are at higher risk for persistent angina, reinfarction and death within several months.
References
Web
1. Luepker RV, Apple Fs et al: Case definitions for acute coronary disease in epidemiology and clinical research studies. Circulation 108:2543, 2003
2. Yerem Yeghiazarians et al, NEJM, Jan 13, 2000
3. Legato MJ.Women’s health, Int J fertile. 1998; 43 (2): 65-72.
4. American Heart Association. Heart and Stroke facts. Dallas, 2003
5. National Heart Attack Alert Program Coordinating Committee Working Group. Educational strategies to prevent prehospital delay in patients at high risk for acute myocardial infarction. Bethesda , National Institutes of Health, National Heart, Lung and Blood Institute, 1997
6. Penque Set al, Women and coronary disease. Am J Crit.Care, 1998; 7 (3): 175-182
7. Canto JG, Shlipak MB et al. Prevalence, clinical characteristics and mortality among patients with myocardial infarction presenting without chest pain
8. Pedoe-Tunstall H et al: Myocardial infarction and coronary deaths in the World Health Organization, circulation 90:583, 1994
9. Jacc 2003: 41:895
10.Circ 2000, 102:2031
11.Elliott M Antman, Braunwald E Heart Disease, 7th edition. ST-Elevation Myocardial Infarction: Pathology, Pathophysiology, Clinical Features and Management
Web References
Fenton, D. E, MD. Acute Coronary Syndrome e Medicine
References for the Patient
Patient should be provided with web address where they can find additional information about heart disease:
National Library of Medicine (www.nlm.nih.gov/medlineplus/healthtopics.html)
National Heart, Lung, and Blood Institute (www.nhlbi.nih.gov/)
American Heart Association (www.americanheart.org)