Big Bang and Biology
Ashley Davidoff MD
The Common Vein Copyright 2012
In the beginning of time there was 0…
In the beginning there was a void, nothing, there was zero
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.8
And Then There was 1…
And then 0 and 1 started linking organizing
…. sometimes in the presence of a facilitator and the two equations (1+1=2, and 1+1= 1 were born)
The poem was written following a discussion between 3 school friends inspired by the concept of the whole being greater than the sum of its parts.
1+1 = 1
Davidoff Abelson and Leveen
In the Beginning
There was 0
Then there was 1
Then 0+1 = 1
1 to the power 0 = 1
1 to the power 1 = 1
1 to any power = 1
Any number to the power 0 = 1
Though 0 to the power 0 is indeterminate
And finally 1 + 1 = 1
Big Bang and the Formation of the Hydrogen Atom … and the beginning of the build up
In the beginning – about 13.7 billion years ago after a big bang, the universe was so hot and then as it began to cool protons neutrons and electrons were born and then the simplest atom – the hydrogen atom was born first, and remains the most abundant atom in the universe contributing to 90% of the atoms in the universe. After about 300 million years the hydrogen atoms started to clump together under the force of gravity generating pressure as they clumped and then heat resulting in nuclear fusion. As a result of nuclear fusion of two hydrogen atoms helium , then lithium was formed and then the rest of the elements up to iron (26 hydrogen atoms) were formed (http://www.physicscentral.com/explore/poster-stardust.cfm) Therefore matter formed creating the stars and then galaxies in the heavens and the table of elements on the earth up to iron. After iron nuclear fusion requires more energy than it produces. The inner core of the earth has a large amount of iron.

Big Bang
|
|
In the beginning – about 13.7 billion years ago there was a big bang and the universe was formed. It was very hot but when it started to cool, electrons, protons, and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff MD 102976p.8s
|
The Birth of the Forces of the Universe and the Beginning of Biology

The Birth of Protons, Electrons, and Neutrons
|
|
When the universe started to cool the electrons, protons and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff 102976pb04b06b01b.8s
|
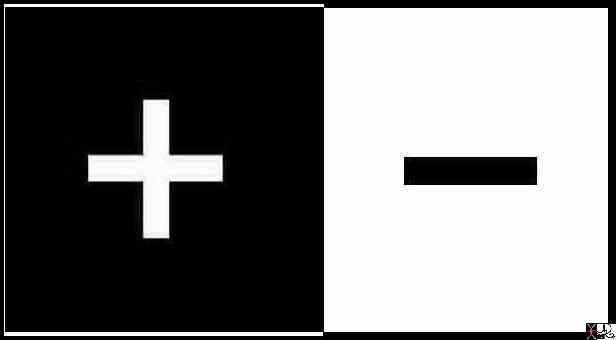
There were two basic forces in nature … positive and negative, and a neutral force, called the neutron.
In the atomic world .the positive is encapsulated in the proton and the negative in the electron while the neutron resides with the proton in the nucleus.
The Primal Electromagnetic Forces
Positive and Negative
|
|
The primal opposites, attracted each other’ like nothing else, forming an unavoidable union, while retaining their individuality and going for the most part their separate ways, but remaining bonded. The first and most abundant union and the building block of all to follow is the hydrogen atom consisting of one electron and one proton.
Copyright 2012 Courtesy Ashley Davidoff MD 84154b04.698
|
When positive and negative interact – it is not a simple interaction. It is not a 1+1 =2 , and is closer to 1+1=1. It is like marriage. Firstly by their union they still retain their individuality, go their separate ways with the electron in orbit and the nucleus staying central, but remain bonded and together they form a new unit.
Evolving Units
When a new unit is formed from its component parts, an interaction has taken place in a given space, in a given moment in time, in a given environment. The new unit has unique characteristics that is not manifest in either of the component parts.
At the time of big bang the electron, proton, and neutron were formed.
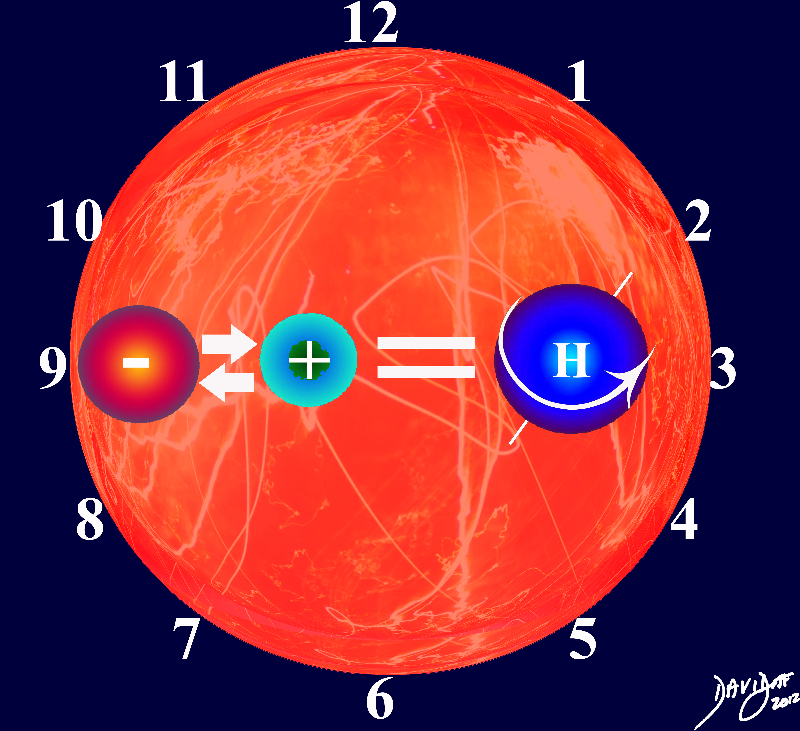
Formation of the Hydrogen Atom
|
|
After the big bang one of the first interactions between the electrons and protons was in the formation of the hydrogen atom An electron (unit 1) interacted with a proton (unit 2) in the heated environment in the space of the universe, at a moment in time, and a new and unique unit 3, called the hydrogen atom was formed. because the interaction resulted in a spinning charge an electromagnetic field was set up and the atom also spun in space in a unique way that was not characteristic of either the proton or the electron.
Copyright 2012 Courtesy Ashley Davidoff MD 102976pb03b04.8s
|
The hydrogen atom is the building block of all else to come.
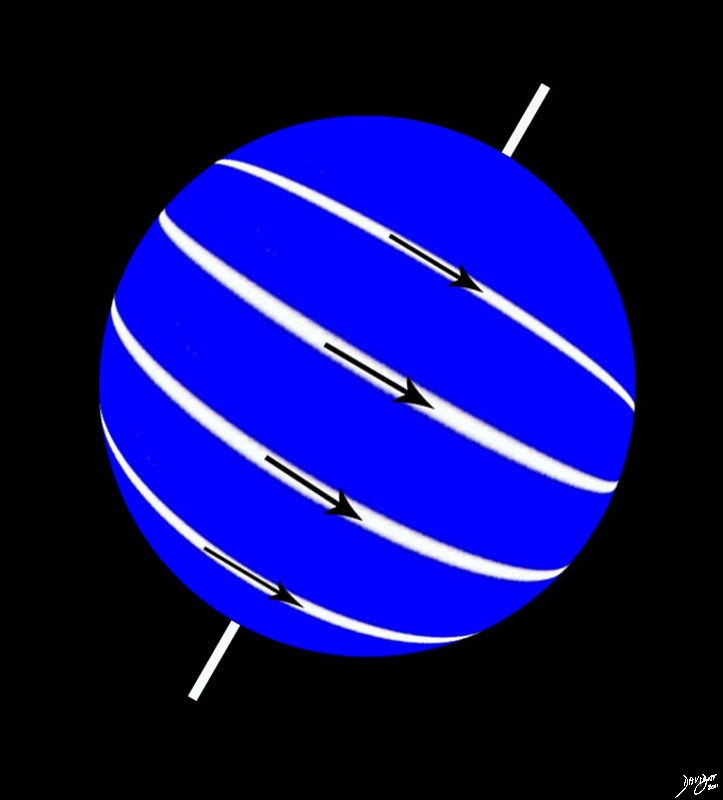
The Hydrogen Atom Spinning Precessing Magnetic Field
Mass, Motion, Space, Time and Electromagnetic Force
|
|
The spinning hydrogen atom introduces us to the elements of mass, motion, space, time, and electromagnetic forces.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b22.8.8s
|
Some Universal Principles
The marriage between the electron and the proton is not a simple one. It is not as black and white as it seems since as a result of the marriage the electron is bound to the nucleus and the proton is bound to the electron. The motion of the electron in an orbit allows it to keep its distance from the proton and prevents the two from crashing and falling head over heels into each other, or nulling each other. The hydrogen atom is set in motion and as a result of the electrical force in motion an electromagnetic field is set up. The spinning of the hydrogen atom forces the atom to be in a certain position in space at a given time as it moves – Voila – the elements of space, time and motion, are manifest in the units. The units have mass and elements of electromagnetic and mechanical forces. The interactions, allow bonding, and new units are established. Uniqueness, dependence and independence of each of the units follows. These are elemental and foundational principles common to all matter- organic and inorganic, biological or otherwise and will be repeated ad nauseum in the following texts.
With the birth of the universe, an infinitismal number of hydrogen atoms were born and let loose to build bigger and better units with their new found expression through mass, time, space, electromagnetic force, and motion.
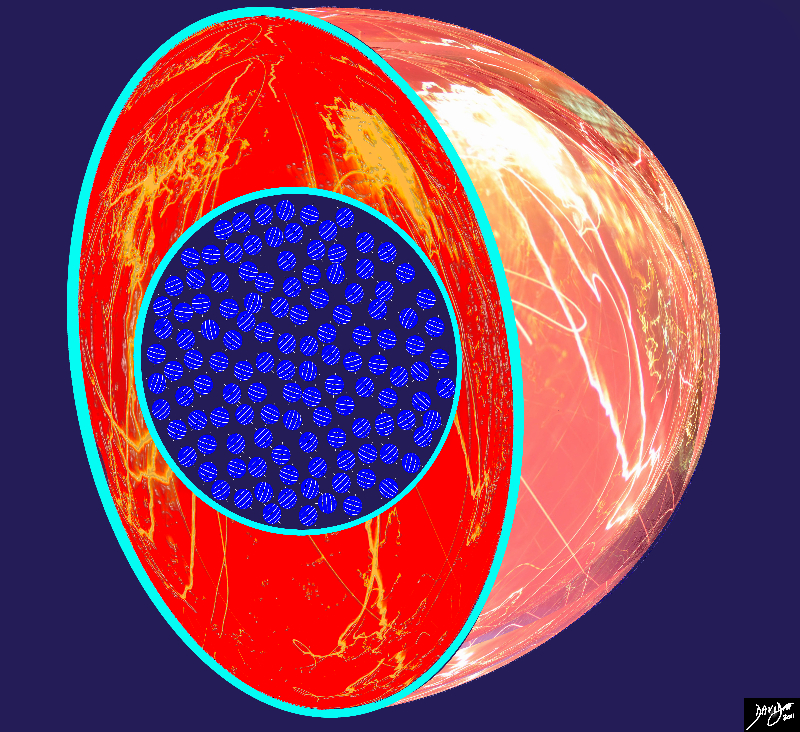
The Birth of the Proton from the Womb of the Hot Hot Hot Universe
|
|
Hydrogen, as simplest and lightest atom, remains the most abundant atom in the universe, and contributes to 90% of all the atoms of the universe.
Courtesy Ashley Davidoff MD 102976pb04b10b06b.8s
|
The electromagnetic forces and the fact that the hydrogen atoms are moving, forced the atoms to come into contact with other hydrogen atoms and random reactions took place in the presence of the given environment in which temperature, mass, motion, space, time, electromagnetic forces, and facilitators were relevant to the reaction and the result was the creation of other atoms and elements.
http://www.chemistry.mcmaster.ca/esam/Chapter_4/section_2.html
The Building of New atoms and Elements
Doubling Up the Forces – Helium
|
|
When two hydrogen atoms combined in a given environment, at a moment in time and space, helium the second lightest and second most abundant element in the universe is produced.
Copyright 2012 Courtesy Ashley Davidoff MD 84154c03.800b01
|
And as we double the units of force we find that there is a relatively weak electric force and another force called a strong force
Fission and Fusion – Strong forces and Electrical forces – (http://www.youtube.com/watch?v=yTkojROg-t8
And as we build up the negative and positive charges and we add neutrons in the mix the units get linked and organized in different ways to create new units with completely different characteristics
As we add units things start to change to a point where electrical forces start to dominate
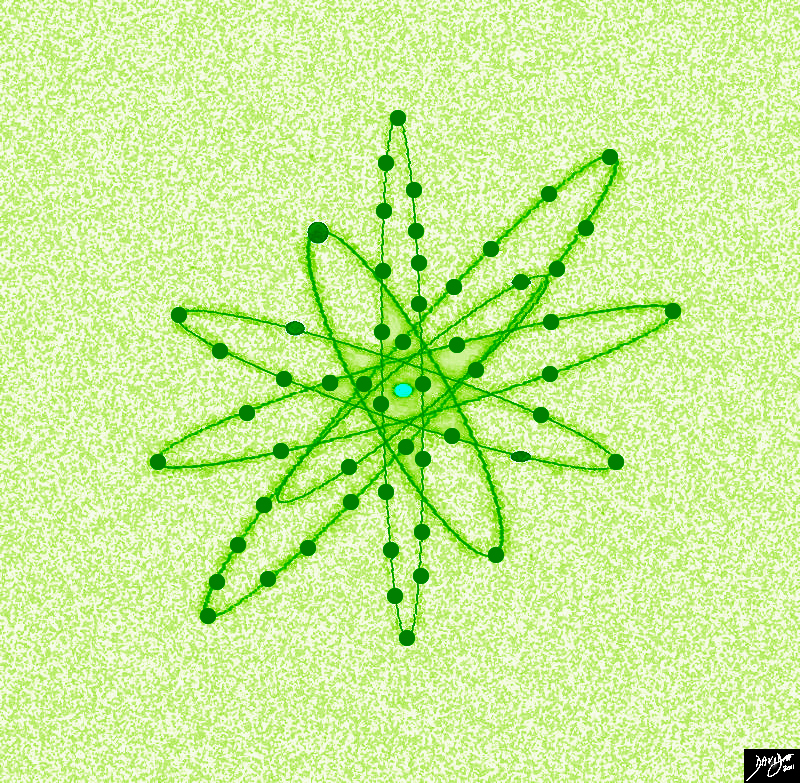
The Atom – The Powerful and Indivisible
This atom has 56 electrons in 6 orbits It is called barium |
|
In this diagram of barium we note the electrons spinning around the nucleus Barium has 56 electrons, 56 protons and 81 neutrons giving it an atomic weight of 56+81 = 137. The electrons atre distributed in the orbits in the following manner 2,8,18,18,8,2.
Copyright 2012 Courtesy Ashley Davidoff MD 45824b13bd10.8s
|
ttp://en.wikipedia.org/wiki/Electron_shell#Number_of_electrons_in_each_shell
The remarkable similarity between the structure of the atom, and the structure of the solar system, leads one to ponder the recurring organizational pattern of things in between. If one considers the atom as a unit and the solar system as a unit then the technique of combining units to build bigger units more powerful than the individual parts allows us to understand in some measure the unifying units to units concept or 1+1= 1
Atoms are the smallest parts of matter that possess the distinctive chemical characteristics of chemicals that we can recognize in our surroundings
If for example split a piece of charcoal, which consists of pure carbon, and break it up into its component parts, it will end up as an atom of carbon that will have the same chemical characteristics as the original charcoal If you were to do the same thing of chopping up an iron molecule till it ends up with characteristic component parts, you would end up with iron atoms that behave in similar fashion as the original iron bar If the chopping continues then the iron particles will be broken up into protons, neutrons and electrons.
If on the other hand we took water and started breaking it up into its component parts then we would arrive at the molecules of water wth 2 hydrogen atoms and one oxygen atom. Individually these would not behave like the water molecule but rather like hydrogen and oxygen individually. Then as they get broken up further into electrons protons and neutrons, they will not behave like oxygen or hydrogen but like protons electrons and neutrons.
When a substance consists of a single type of atom it is called an element. There are 92 different elements found naturally on the earth .
A substance that is made up of more than one atom is called a compound.
And atoms of same type link and organize and they form an element
Formation of an Element
|
|
units person body atom solar system universe TCV Davidoff art concept
Copyright 2012 Courtesy Ashley Davidoff MD 45827b10.800
|
and then there are now only 118 of them. The elements are categorized into the groups of alakali metals, alkali earths, halogens, noble gases.
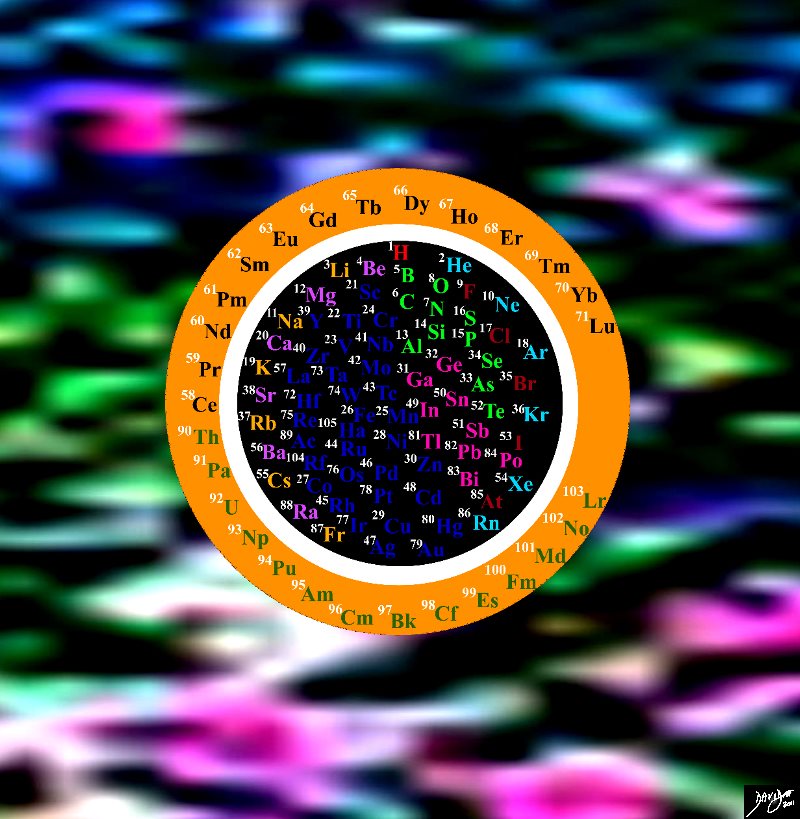
The Elements
|
|
The Elements Now up to 118 of them Periodic Table in the Round 108485pb20.8s
Copyright 2012 Courtesy Ashley Davidoff MD 108485pb20.8s
|
The atoms also link to different atoms to form molecules
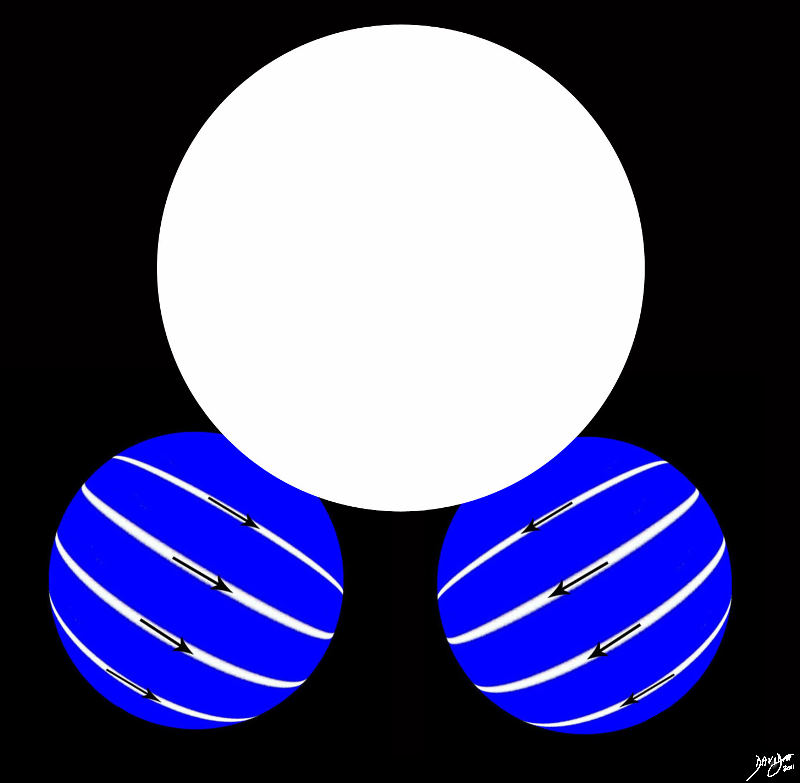
Two Hydrogens and One Oxygen
Water Molecule
|
|
The Molecule Two Hydrogens and an Oxygen Water Davidoff art
Copyright 2012 Courtesy Ashley Davidoff MD 13451b23.8s
|
So what about biology
The Units of Biology
Fundamentals of life:
atomic particles —>molecules—> macromolecules (DNA, RNA)—> self assembling macromolecules—> lipid bilayer formation—> lipid bilayer encapsulating self assembling macromolecules—>compartmentalization—> evolution of those compartments with respect to environment—> replicating cells—>formation of basic bacteria in hot springs—> formation of eukaryotes—> evolution of the cells to organize into tissues—> into organs—>then into living creatures
Carbohydrates fats and proteins
http://en.wikibooks.org/wiki/A-level_Biology/Biology_Foundation/biological_molecules
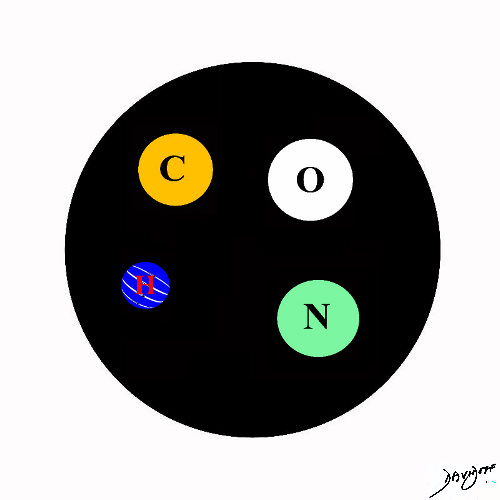
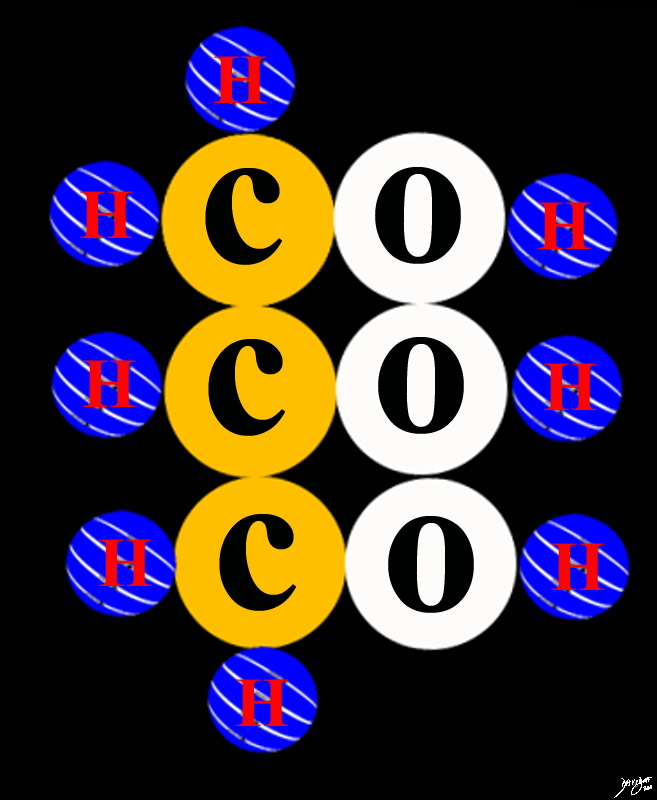
Hydrogen Oxygen Carbon Nitrogen
The Most Common Building Blocks of Biological Materials
|
|
The Most Common Building Blocks of Biological Materials
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.81
|
Carbohydrates
Carbohydrates (hydrates of carbon) are organic compounds that usually contain carbon hydrogen and oxygen in the ratios: 1 Carbon: 2 Hydrogens: 1 Oxygen.

Carbohydrates
C:2H:O
|
|
Carbohydrates Carbohydrates (hydrates of carbon) are organic compounds that usually contain carbon hydrogen and oxygen in the ratios: 1 Carbon: 2 Hydrogens: 1 Oxygen.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.83s
|
The type of carbohydrates include monosaccharides disacharrides
A monosacharride such as glucose is linking and organization to create something bigger and more powerful than the individual parts
Molecules molecules and then macromolecules
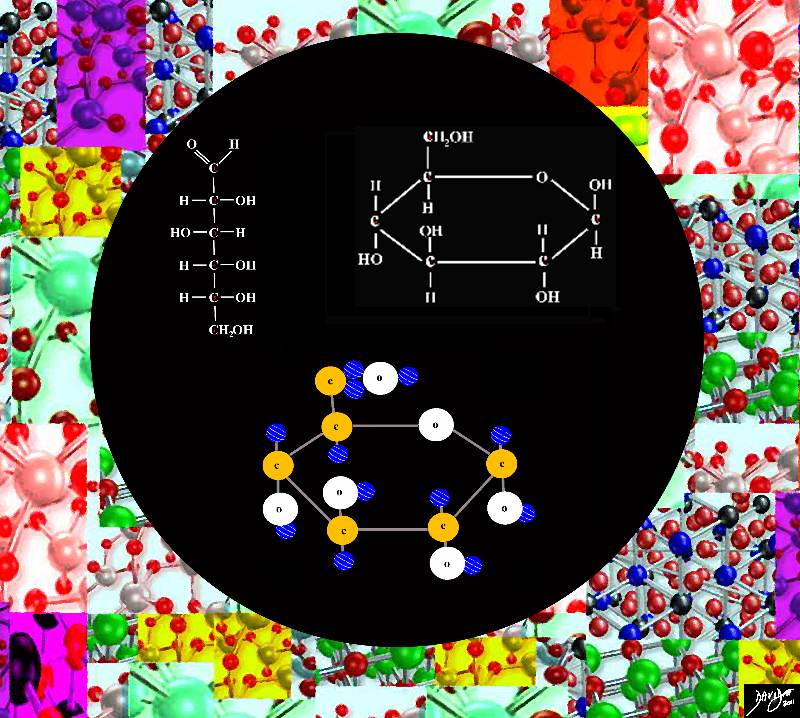
Carbohydrate Monosaccharride Glucose
|
|
Carbohydrate monosaccharide glucose
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf02.8s
|
Lipids triglycerides eg fat and oils
Sterols – Choletserol Vit D and Sex hormones
Phospholipids eg lecithin

Fats A Combination of C H and O
|
|
Fats A Combination of C H and O
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.82s
|
http://www.youtube.com/watch?v=36tlBLwJUxQ
http://www.youtube.com/watch?v=84MvQ9appxQ triglcerides
http://www.youtube.com/watch?v=fCfMH2MT-Is phospholipids
http://www.youtube.com/watch?v=mpJYcFv9MWk&feature=related
95% of the lipids that we eat and 99% of the lipids in our body are triglycerides
Three fatty acids and a glycerol
All fats are derivatives of fatty acids and glycerol. They, like the carbohydrates are created from a combination of carbon hydrogen and oxygen which link and organize to form a fat soluble compound. The triglycerides consist of one unit of glycerol and three units of fatty acids
The fatty acid is a usually a long chain of even number of carbons (usually between 4 to 28 each carbon allowing for 4 bonds. When there are fewer than 6 carbons it is called a short chain fatty acid. When there are 6-12 carbons it is called a medium chain and when there more than 12 carbons it is called a long chain fatty acids.
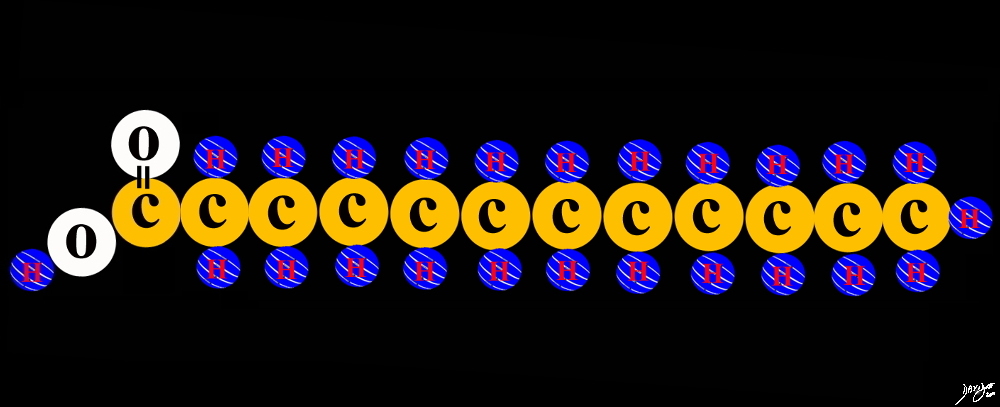
Fatty Acid
|
|
fatty acid
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks
|
When the carbons are linked to each other by single bonds then ther are two hydrogens per carbon and the fatty acid is said to be saturated with hydrogen atoms. When there is a double bond between two carbons then each carbon in the double bond only has room for 1 hydrogen each and the fatty acid is unsaturated with hydrogen atoms
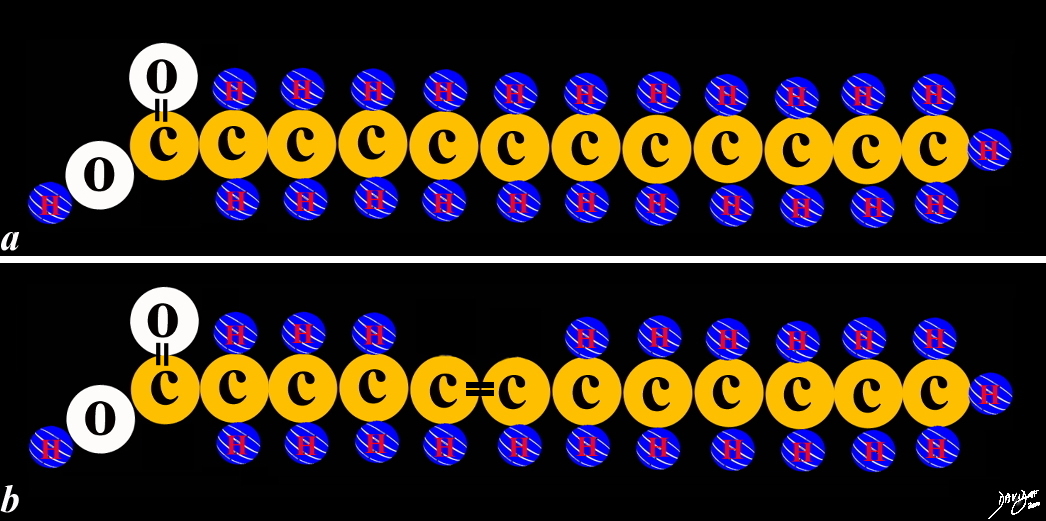
70983.4kf09L.1ks01Ls
|
|
The unique linking and organization of the carbons hydrogens and oxygen creates unique products or metabolites, This linking is not indiscriminate. There are rules that the units have to abide by in order that organization can take place. An obvious rule in this instance is that the carbon can only mintain 4 bonds A double bond takes up two of the availablke 4 . Since each of the bonded carbons use up 2 bonds with the double bond, and are connected to another carbon on each side and threfore they only have room for one hydrogen each.
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks01Ls
|
Enter another molecule called glycerol – a type of alcohol with a hydroxyl group on each of its three carbons
When glycerol links and organizes with with 3 fatty acids a new product is born called triglyceride
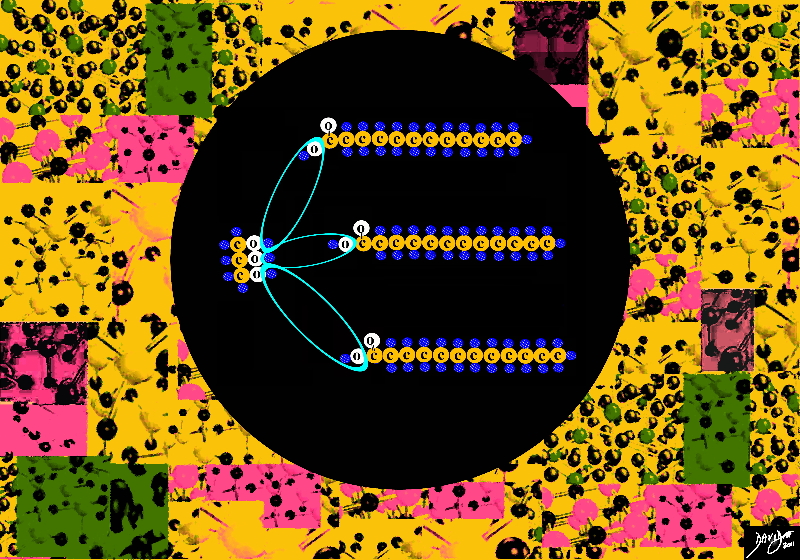
Triglycerides
|
|
The bond or link of the OH groups on the glycerol side links with the carboxyl group (carboxylic acid) OH + COOH
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf08b01.8s
|
Proteins
Proteins are polymers constructed from a series of 20 different amino acids. This is the linking part.
An amino acid is composed of an amine linked to an acid
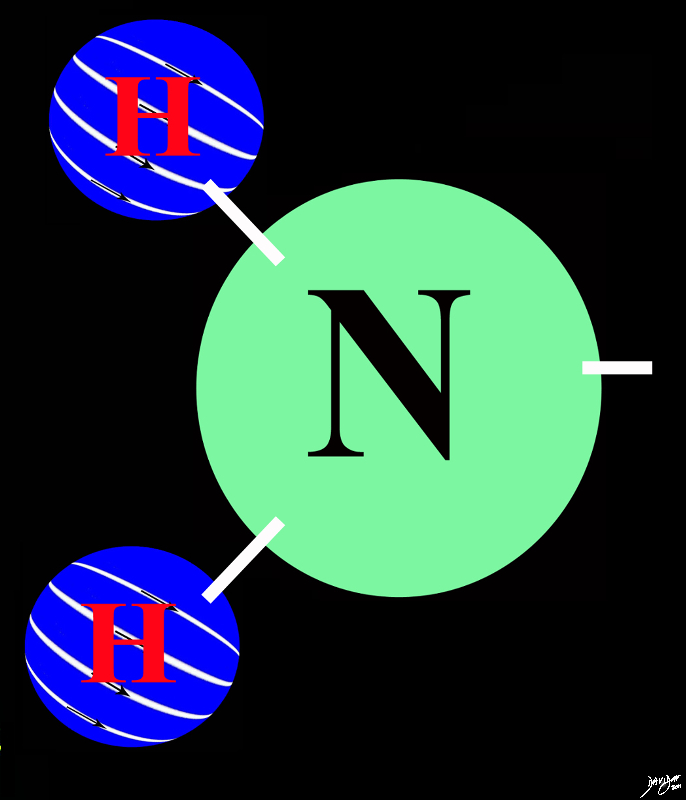
Amine NH3 proteins
|
|
Amine NH3 proteins
Alpha Carbon – The Link
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a02b.8s
|
The organizing part is the a specific sequence specified by the gene resulting in the primary structure.
As a result of the specific linking and organizing a secondary structure results which is a specific coiling of the structure in a unique 3D manner There are two basic types of secondary structure The alpha helix and the beta pleated sheet based on the hydrogen bonds that occur at regular intervals
The tertiary structure is a result of linking of side chains of various amino acids
When other proteins join a quaternary structure results
Amines N HH
Amino acids carbon
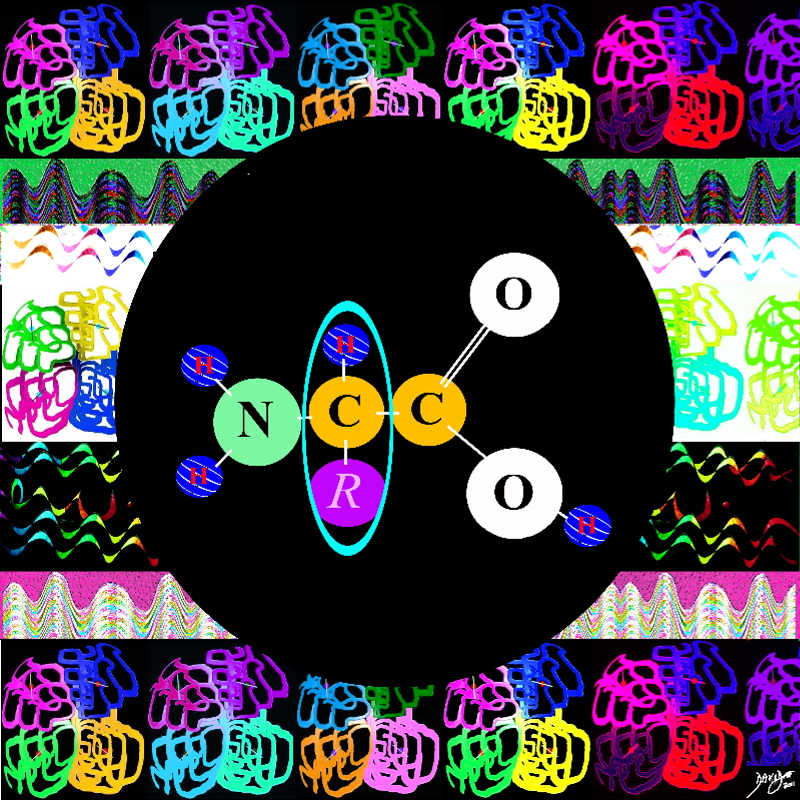 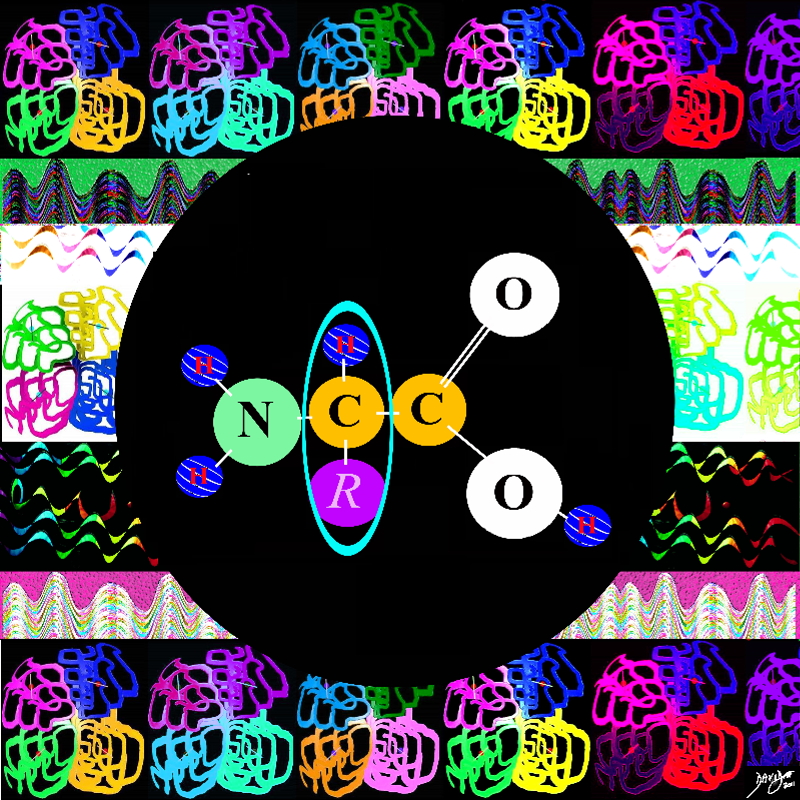
Amino Acid – building block of protein
|
|
Alpha carbon the connector with the “R” representing something else eg hydrogen or CH3 big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a03.8s
|
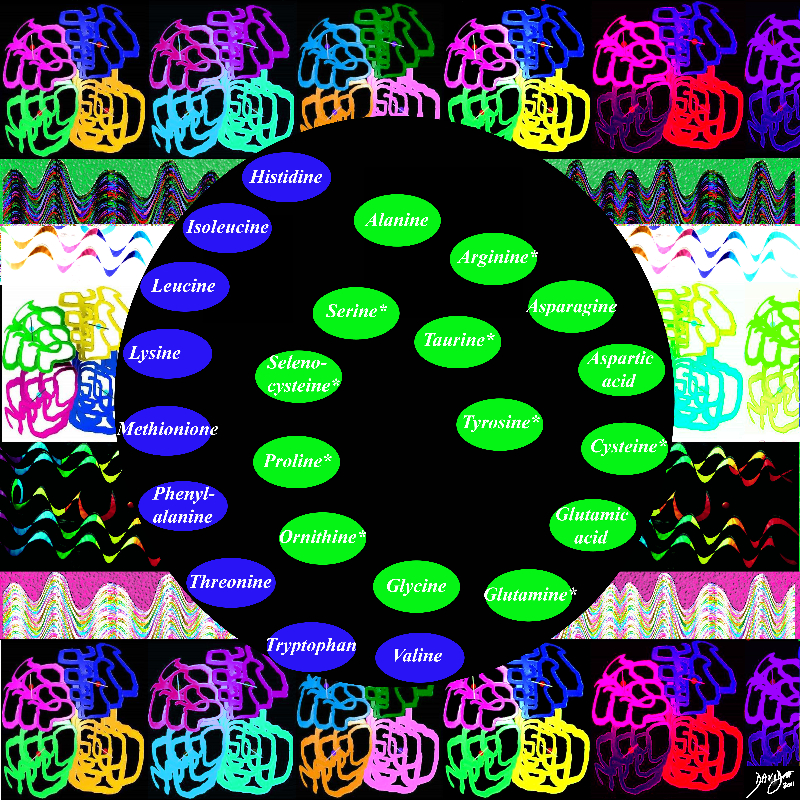
The 20 Amino Acids
|
|
The 20 amino acids
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c13iL.8s
|
The 20 amino acids form polypeptides and polypeptides form proteins
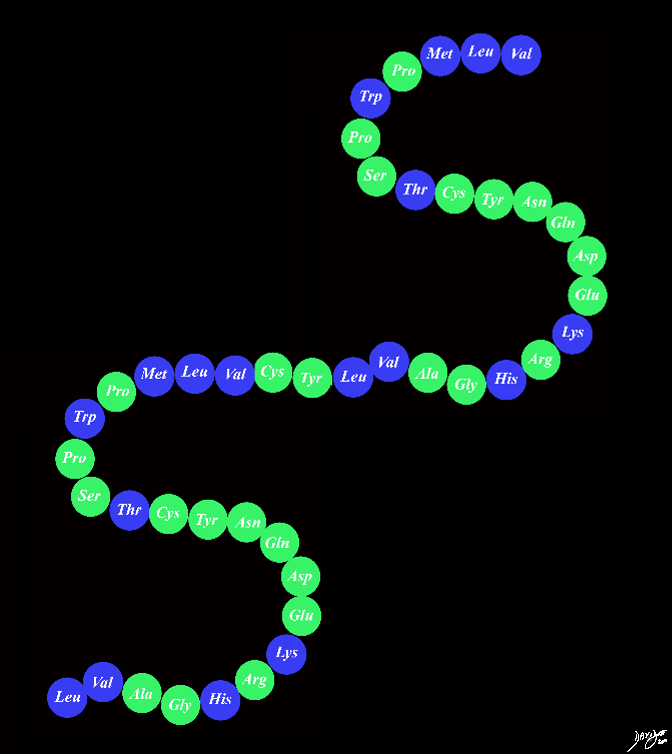
The Protein with Specific Shape
|
|
The Units Link The physical shape of the proteins is their most unique characteristics aspect that allows functional specificity http://www.youtube.com/watch?v=lijQ3a8yUYQ
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.45.8s
|
Making Biological Materials from Proteins
Primary Structure

alpha helix and beta pleated sheet protein
|
|
alpha helix and beta pleated sheet protein
Copyright 2012 Courtesy Ashley Davidoff MD 108247b01.8s
|
Hydrogen bonds between the amino acids determine whether the secondary proteins particpate in almost every aspect of the cell structure and function
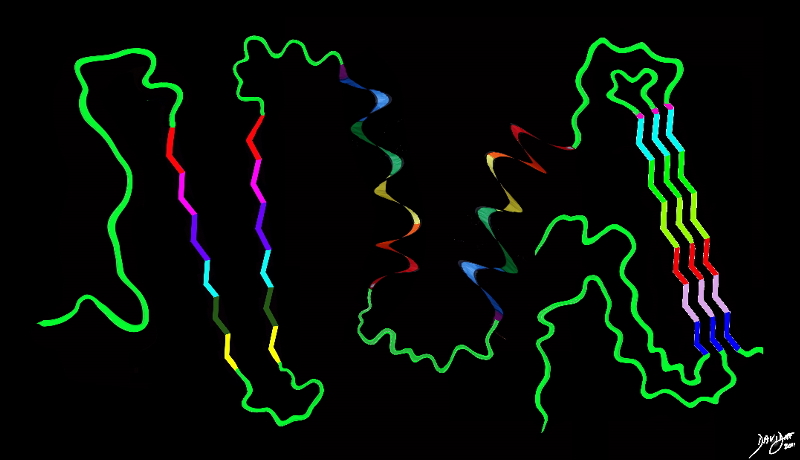
3D protein Shapes in 2D
|
|
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.8.8s
|
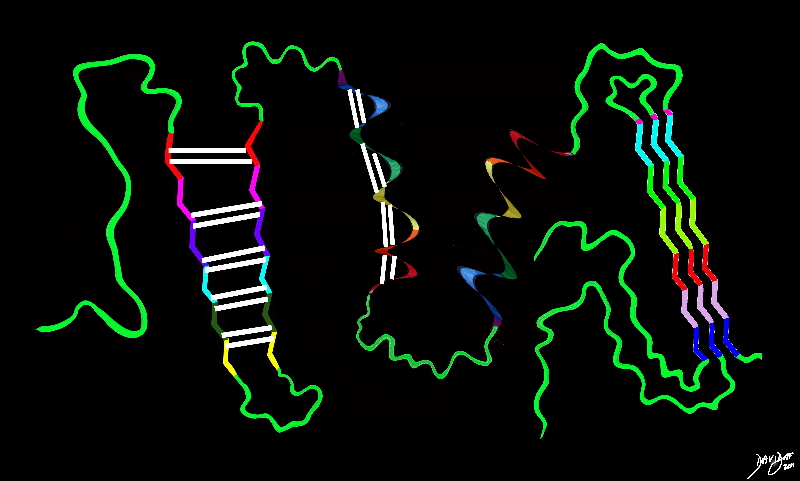
108246b04b09b.8s
|
|
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09b.8s
|

Complex Shapes of Protein Molecules
|
|
complex shape 3D protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.84.8s
|
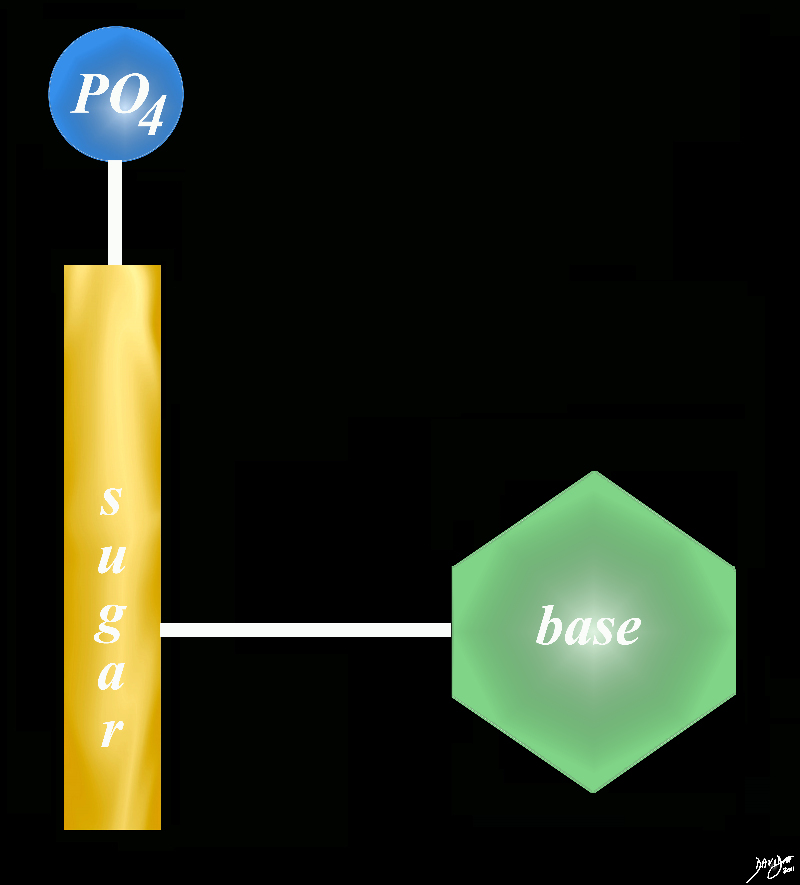
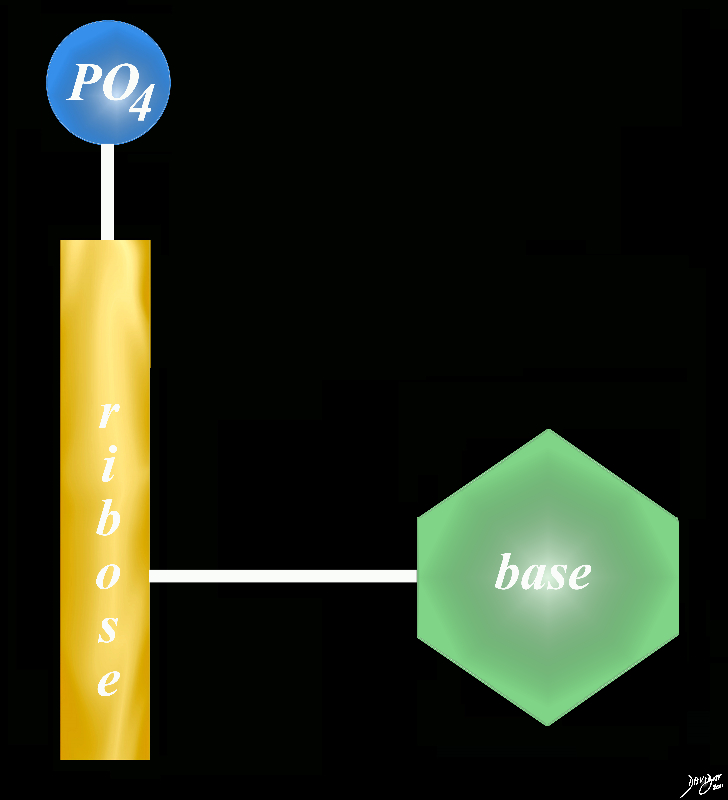
phosphate sugar and a base = nucleotide
|
|
phosphate sugar and a base = nucleotide
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L.8s
|
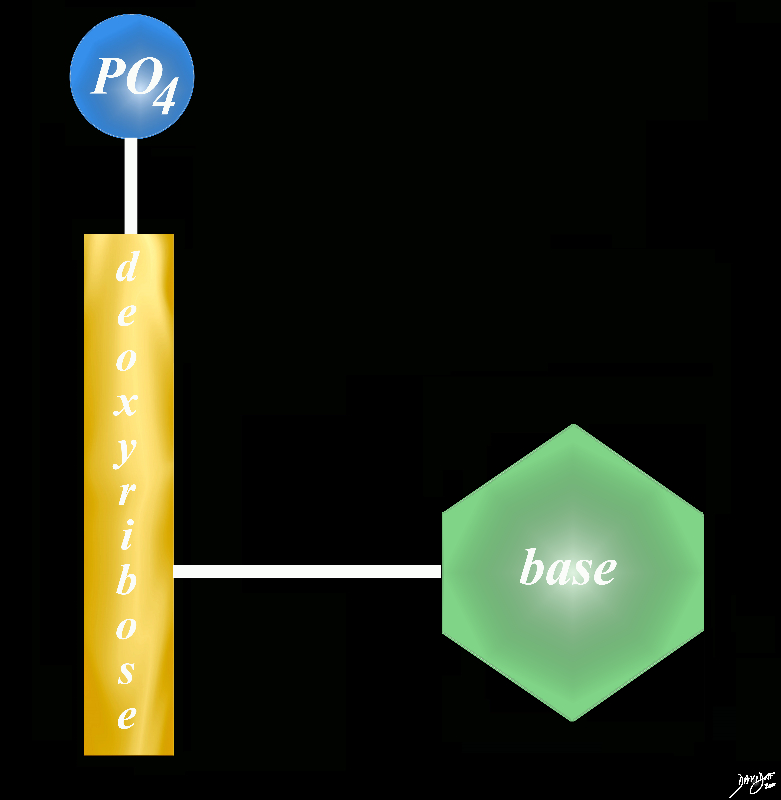
DNA – Sugar is Dexyribose
|
|
DNA A base is a a substance that can accept hydrogen ions (protons) or more generally, donate electron pairs. The base in the nucleotide is a nitrogen containing base = nitrogenous base
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01.8s
|
A base is a a substance that can accept hydrogen ions (protons) or more generally, donate electron pairs.
The base in the nucleotide is a nitrogen containing base = nitrogenous base
DNA
3 parts Phosphate group sugar (deoxyribose)and one of four bases adenine guanine cytosine thymine
sugar phosphate = hand rails together thy form a nucleotide
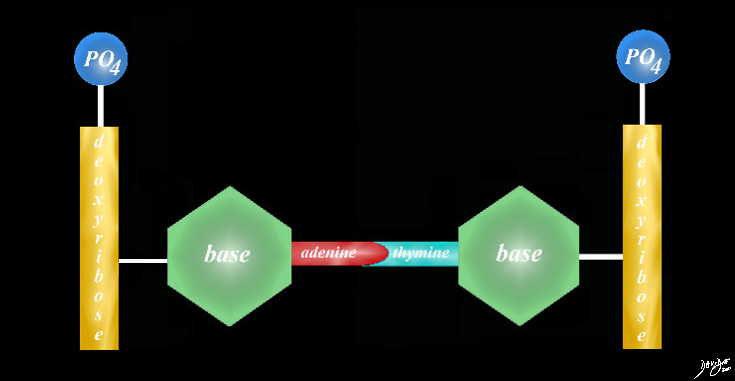
Bonnding of Two BAses Thymine and Cytosine
|
|
the bases will bond via hydrogen bonds adenine only to thymine and cytosine only to guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc01.83s
|
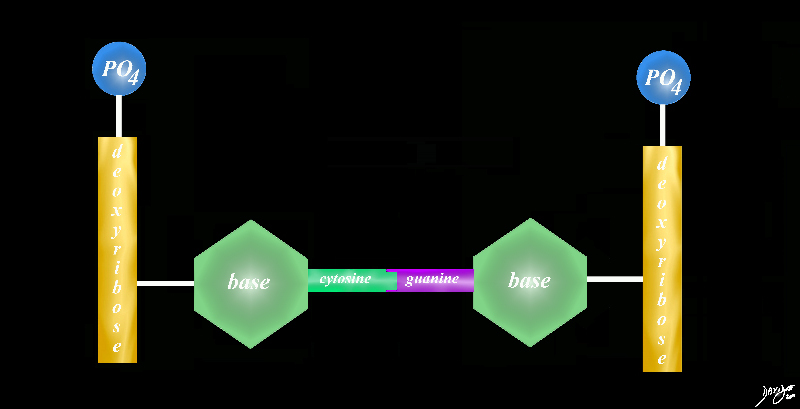
DNA sugar base protein cytosine guanine |
| DNA sugar base protein cytosine guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc02L.81s
|
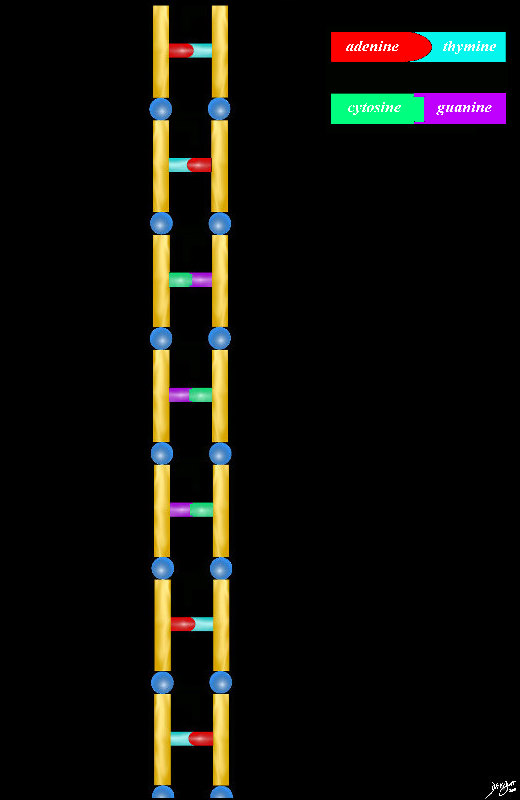
78612pb01.58kb03b08.3k.81s
|
|
chromosomes genes double spiral DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb03b08.3k.81s
|
the basic pairs never change the order of the pairs varies from one species to the next
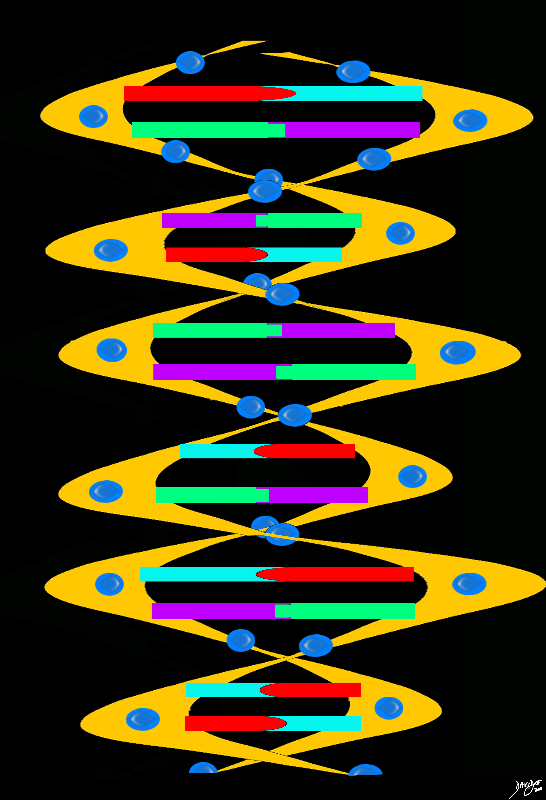
Double Helix
|
|
chromosome genes double helix DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.67kb06.82s
|
RNA
Made of nucleotides
Ribose as a pentose sugar
Uses Uracil and not Thymine
phosphate
RNA Ribose is the Sugar
|
|
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L02.8s
|
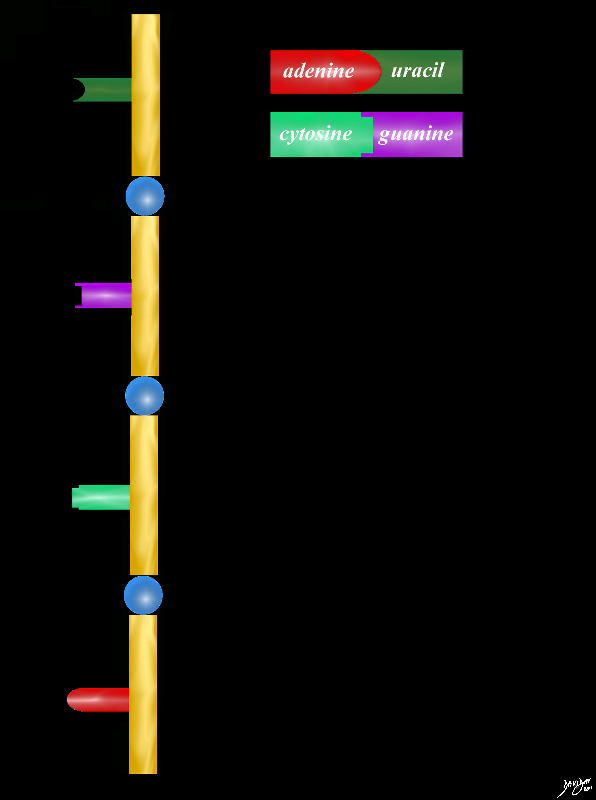
78612pb01.58kb05L.8s
|
|
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L.8s
|
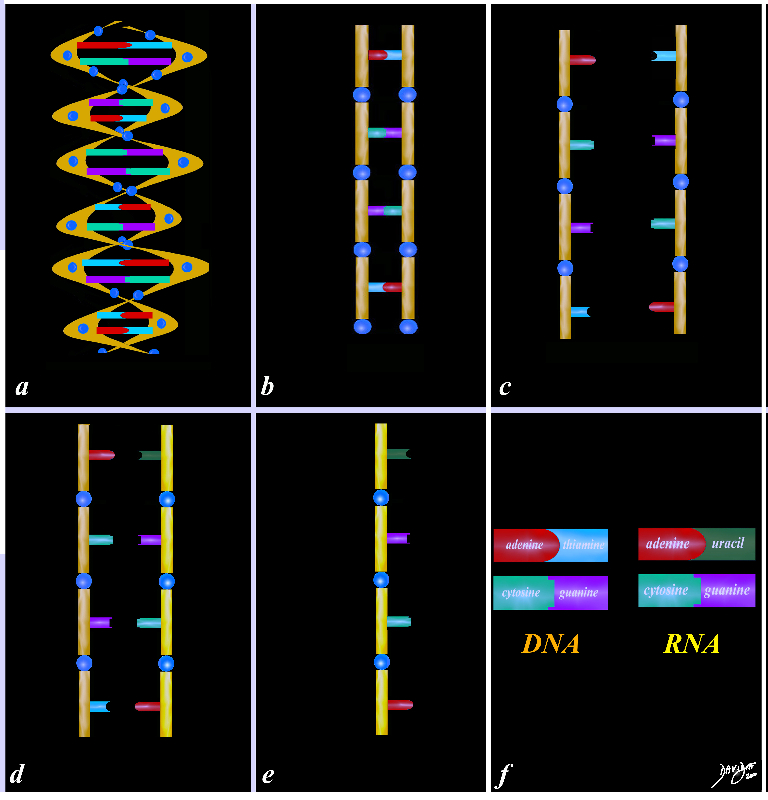
78612pb01.58kb05L04.8s
|
|
DNA double helix uncoil transfer encryption messenger messenger RNA transcription RNA sugar ribose base protein cytosine guanine adenine tymine uracil
a)= DNA in spiral b) uncoling c) separating d) RNA match up e) RNA encoded f) base pairs in DNA and RNA
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L04.8s
|
Phosphate
A U or T
G to C
Self Assembling Macromolecules
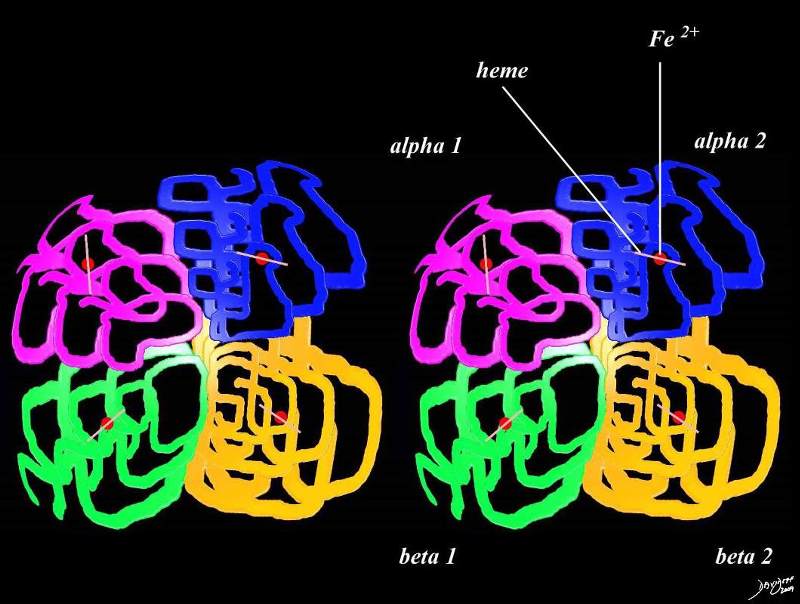
Hemoglobin
|
|
Hemoglobin consists mostly of proteins aka the 4 “globin” chains. The hemoglobin molecule is composed four globular protein subunits. There is an alpha chain (alpha 1 and alpha 2) and a beta chain (beta 1 and beta 2) All the proteins stick together These proteins, are made up of sequences of amino acids. Both proteins must be present to enable the hemoglobin to carry and release oxygen normally. Before birth, the beta protein is not present. Instead a gamma protein is present in the fetus but is replaced by the beta chain at birth. Each protein chain tightly associated with a non-protein heme group. A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic ring, known as a porphyrin. The iron ion, which is the site of oxygen and C02 binding In binding, oxygen temporarily oxidizes (Fe2+) to (Fe3+)
Copyright 2012 all rights reserved Courtesy Ashley Davidoff MD 88528b03c02.81s
|
The Making of Membranes Lipid Bilayer Formation
All cells and organelles are bound by a cell mebrane
Cell mebranes are made of fats and proteins
The fats (phospholipids) form a double layer and the proteins which are contained within the membrane
http://www.google.com/imgres?hl=en&sa=X&biw=961&bih=1019&tbm=isch&prmd=imvns&tbnid=y17T_rCAQqHrgM:&imgrefurl=http://www.madsci.org/posts/archives/2006-12/1164999854.Bc.r.html&docid=ZCWc745u6Fyf-M&imgurl=http://www.madsci.org/posts/archives/2006-12/1164999854.Bc.1.gif&w=580&h=450&ei=HksKT_CmCoTX0QGLy_ygAg&zoom=1&iact=hc&vpx=301&vpy=569&dur=8317&hovh=198&hovw=255&tx=128&ty=135&sig=100078742493931344069&page=1&tbnh=144&tbnw=186&start=0&ndsp=20&ved=1t:429,r:13,s:0
http://www.indiana.edu/~oso/Fat/Definitions.html
Phospholipids consist of a head and a tail
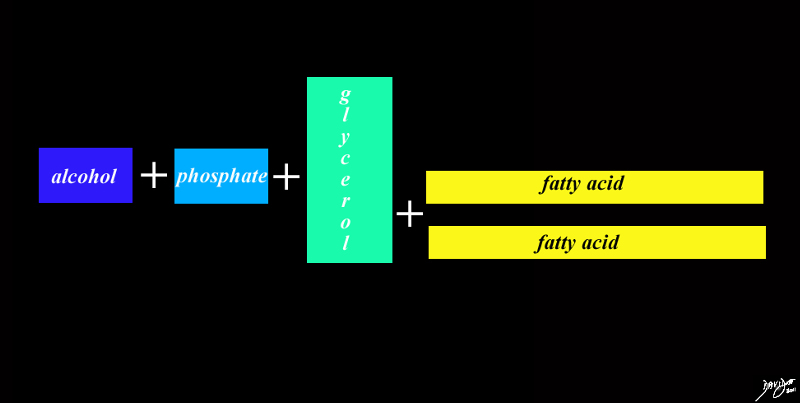
Chemical Components of Cell Membranes
|
|
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b07.84s
|
The head universally contains an alcohol phosphate and glycerol and the tail two fatty acids
The backbone of the head is glycerol
An example is lecitihin
The head which is hydrophilic is made of
a- choline component
b- phosphate component
c- glycerol
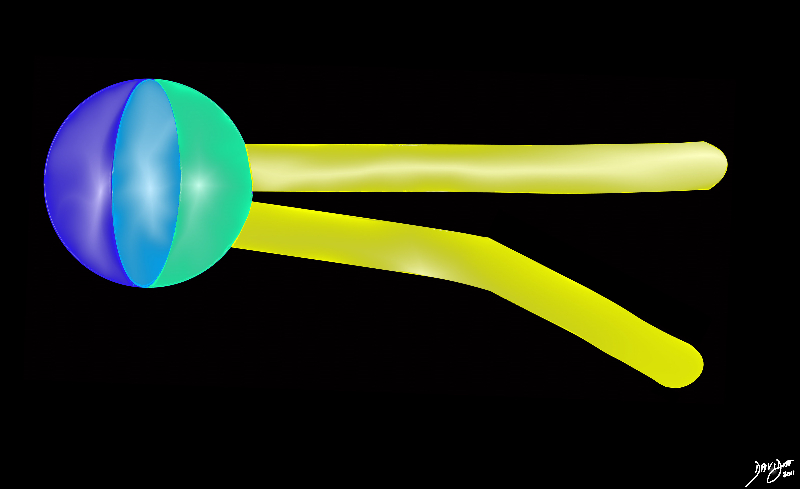
Head and Two Tails of a Phospolipid Membrane
|
|
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
head = dark blue (alcohol) + light blue (phosphate) +green (glycerol
tail = yellow (2 long chain fatty acids)
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j05.8s
|
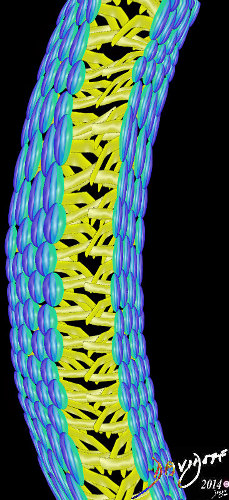
Linking and organizing with hydrophillic head facing outside environment, two fat soluble hydrophobic layers facing each other, and the other layer of hydrophilic heads facing the inside environment of the cell.
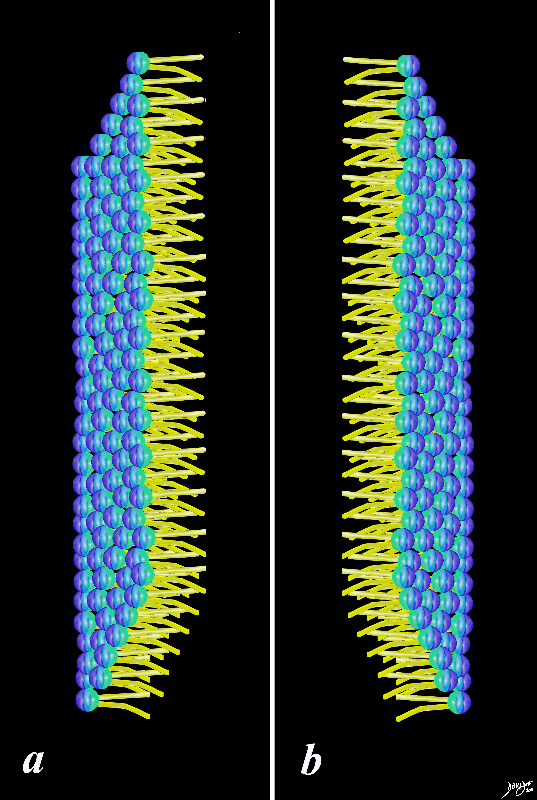
Two Layers Facing Each Other
|
|
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b03.8s
|
Two LAyers Hydrophobic on the Inside and Hydrophilic FAcing Our and In to the Cytoplasm
|
|
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10b.8s
|
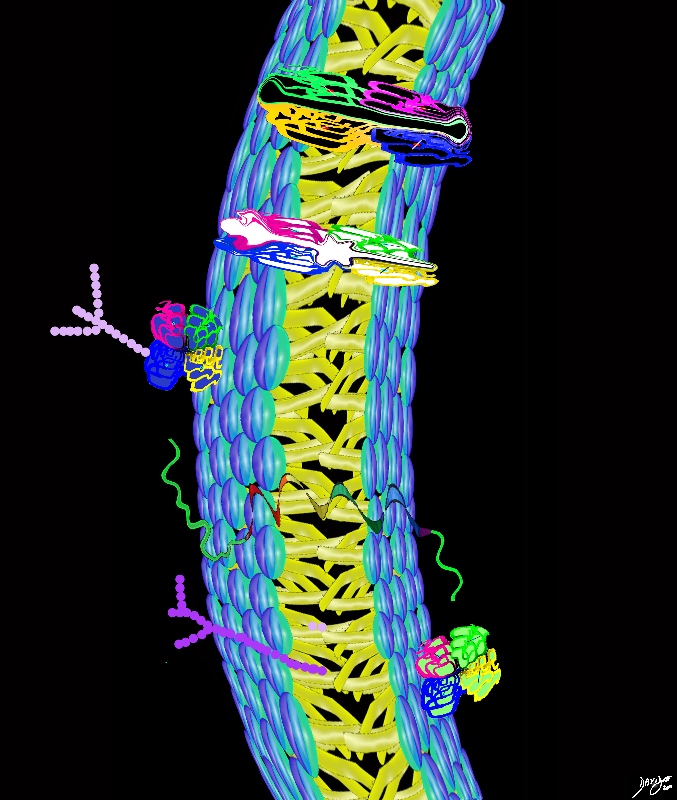
The cell membrane has to add more units to reach full functionality
Firstly proteins
are needed identify the cell – a tag if you will to say who the cell is
enable interaction with external and internal environment
allowing products to enter and products to leave the cell
Movement is by
osmosis
diffusion
pump
electrical charge
Types of functionality demanded of the cell membrane and how proteins assist
surface protein markers – identifies the cell
receptor proteins bind to messengers or hormones that change permeability of a cell
ion channels Charged pores allow molecules to pass through the cell membrane as long as they are the correct size and charge
membrane pumps uses energy (ATP) to open and close gates – Classical is the Sodium potassium pump
carrier proteins Bind to a solute and carry it into the cell
trigger the formation of another messenger and then activate enzymes in the cell to accelerate chemical reactions
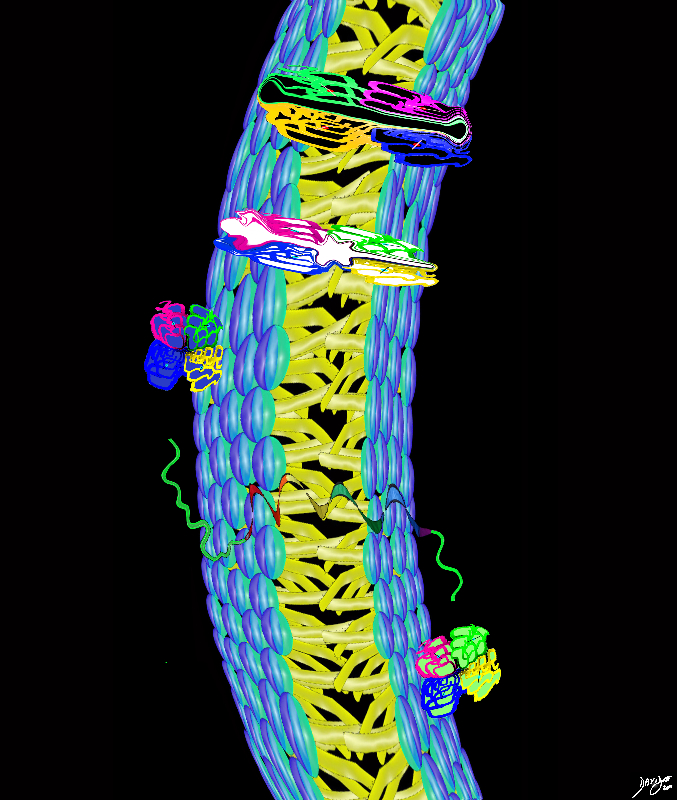
Proteins in the Cell Membranes
|
|
Proteins Transmembrane protein (Globular Protein Channel Protein) Alpha helix Protein Surface protein (peripheral protein) Gated Channel cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc01.8s
|
Carbohydrate Function
Carbohydrates in the Membrane
|
|
carbohydrates Glycoproteins and Glycolipids cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc03.8s
|
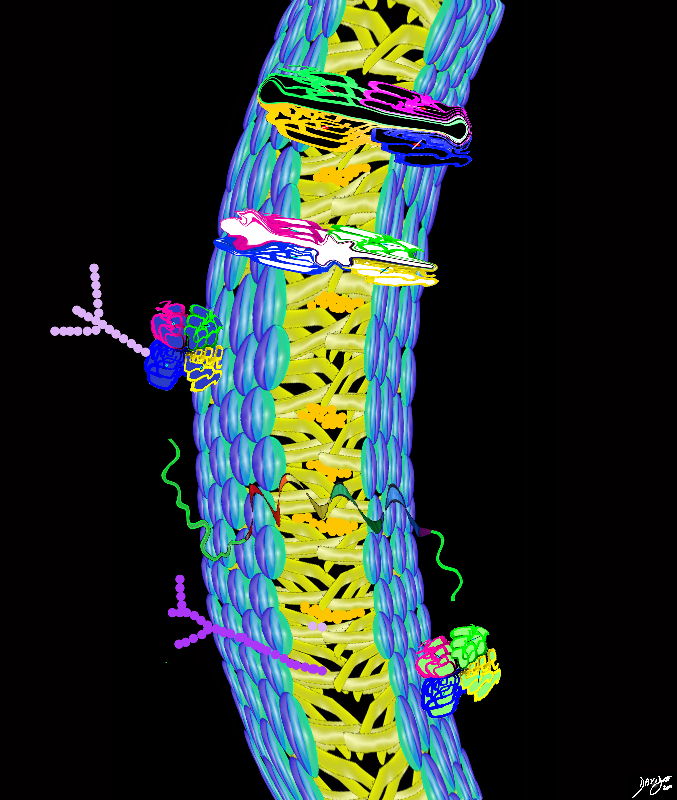
Cholesterol Acts as a Padding between the Phospholipd Tails
|
|
Cholesterol Padding Other fats in the membrane Cholesterol molecules – prevent the phospholipid tails from sticking to each other cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc04.8s
|
Carbohydrate chains
Lipid BilayertEncapsulating Self Assembling Macromolecules
Compartmentalization
Evolution of the Compartments with Respect to Environment
Replicating Cells
The parts
Cytoplasm soup of proteins fats carbohydrates nucleic acids ions waterbody
The Nucleus |
|
cell organelles parts membranes nucleus chromatin cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 82835pj.8s
|

The Endoplasmic Reticulum
|
|
cell organelles parts membranes endoplasmic reticulum cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.68b02k.8s
|

Golgi Apparatus
|
|
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108274b13.8s
|

Mitochondrion
|
|
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus mitochondrion cytology normal
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.61b.8s
|
The mitochondrion 3D video Harvard – All Visual http://www.youtube.com/watch?v=4OgCMzvjaws
108275b04.75bk.8s.jpg
The Cell – Nucleus Organelles and Cytoplasm
|
|
cell organelles parts membranes endoplasmic reticulum golgi apparatus mitochondrion proteins phospholipids ribosomes lysosomes water cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.75bk.8s
|
Connects cells to each other and form tissues
In the world of language the single and the simple is the letter, while in the world of music and painting it is the note, and the brushstroke respectively. The letter moves on to the word, the note to the bar, and the brush stroke to an object in the painting. In biology the cell is the basis of the organ and organism. The Mozarts, van Goghs, Einsteins and Shakespeare’s of the world, organized connected and integrated the units in such a way so as to create a genius result – certainly one that is more powerful than the individual parts. In biology, years of evolution have slowly crafted the human body into a miraculous machine which we have the good fortune to own, honor , learn about, and opportunity to unravel and understand its mysteries in the context of the medical world.
The yearning to be “one” drives us, whether we conciously know it or not. The atom thinks it is indivisible (and probably invincible) but it also has smaller parts. The atom by itself is powerless unless it combines with similar atoms to make up molecule. This same feeling of power and individuality comes to the cell, which for practical purposes, is the smallest living individual and independent unit in biology. It is made up of smaller parts of macromolecules that combine to form microstructures such as the ribosome, endoplasmic reticulum, and the nucleus. All parts in combination make up the cell, giving it singularity and oneness. The cell is powerless without the tissue, and so it combines with similar cells to humble itself before the tissue. Although the tissue claims individuality, it too seeks combination with other tissue as it searches for oneness in the organ. The seeming accomplishment of unity continues through the body to the individual, the family, the community of the village, the community of the nation and the community of the world – and even the solar system, which bears uncanny structural similarity to the atom. The solar system probably thinks it is the end of the world, but the universe has a surprise in store. At this writing, the universe resides at on the one end and the atom on the other – both claiming singularity – but we have to suspect there may be something beyond both of them.
size of structure
coffee bean = 12 X 8mm
grain of rice = 8 X 2.5mm
sesame seed = 3 X 2mm
grain of salt = .5mm
ameba = 500 microns
paramecium = 210 X 60 microns
human ovum =130 microns
photoreceptor =100 X 2.5 microns
sperm = 60 X 5 microns
skin cell = 30 microns
red cell = 8 microns
X chromosome = 7 microns
baker’s yeast = 3 X 4 microns
E Coli bacterium = 3 X .6 microns
mitochondrion = 4 X .8 microns
lysosyme = .1 micron
measles virus = 220 nanometers
influenza virus = 130 nm
hiv virus = 130 nm
coated vesicle = 90 nm
hepatitis virus = 45nm
rhinovirus = 30nm
ribosome = 30nm
antibody = 12 nm
tRNA = 7nm
phospholipid = .9 X 3.4nm
methionine = 1100 X 700pm
adenine = 1300 X 760pm
glucose = 900pm
water molecule = 275pm
carbon atom = 340pm
Carl Sgan Explores the Cell http://www.youtube.com/watch?v=UT_HQ6l72Og
Biologic Units
The Cell
So our bodies contain 7X1027 atoms and of those approximately 4.2X1027 hydrogen atoms. Hydrogen is big bang dust.
How do the atoms get from the big bang to the body?
Miller Urey Experiment http://www.chem.duke.edu/~jds/cruise_chem/Exobiology/miller.html
Botany More Complex than the Cosmos
The construction of the universe is certainly very much easier to explain than is that of the plant.
Georg Christoph Lichtenberg
Aphorisms, trans. R. J. Hollingdale (1990), 119.
Atoms and Sparrows
Who sees with equal eye, as God of all,
A hero perish or a sparrow fall,
Atoms or systems into ruin hurl’d,
And now a bubble burst, and now a world.
Alexander Pope
‘An Essay on Man’ (1733-4), Epistle I. In John Butt (ed.), The Poems of Alexander Pope (1965), 507.
Understanding Physics and Biology Together – The Goal (a)
Yet is it possible in terms of the motion of atoms to explain how men can invent an electric motor, or design and build a great cathedral? If such achievements represent anything more than the requirements of physical law, it means that science must investigate the additional controlling factors, whatever they may be, in order that the world of nature may be adequately understood. For a science which describes only the motions of inanimate things but fails to include the actions of living organisms cannot claim universality.
Arthur Holly Compton
The Human Meaning of Science (1940), 31.
Understanding Physics and Biology Together – The Goal (b)
[Certain students] suppose that because science has penetrated the structure of the atom it can solve all the problems of the universe. … They are known in every … college as the most insufferable, cocksure know-it-alls. If you want to talk to them about poetry, they are likely to reply that the “emotive response” to poetry is only a conditioned reflex …. If they go on to be professional scientists, their sharp corners are rubbed down, but they undergo no fundamental change. They most decidedly are not set apart from the others by their intellectual integrity and faith, and their patient humility in front of the facts of nature…. They are uneducated, in the fullest sense of the word, and they certainly are no advertisement for the claims of science teachers.
Anthony Standen
In Science is a Sacred Cow (1950), 18-19
The Common Vein
Hubble’s observations suggested that there was a time, called the big bang, when the universe was infinitesimally small and infinitely dense. Under such conditions all the laws of science, and therefore all ability to predict the future, would break down. If there were events earlier than this time, then they could not affect what happens at the present time. Their existence can be ignored because it would have no observational consequences. One may say that time had a beginning at the big bang, in the sense that earlier times simply would not be defined. It should be emphasized that this beginning in time is very different from those that had been considered previously. In an unchanging universe a beginning in time is something that has to be imposed by some being outside the universe; there is no physical necessity for a beginning. One can imagine that God created the universe at literally any time in the past. On the other hand, if the universe is expanding, there may be physical reasons why there had to be a beginning. One could still imagine that God created the universe at the instant of the big bang, or even afterwards in just such a way as to make it look as though there had been a big bang, but it would be meaningless to suppose that it was created before the big bang. An expanding universe does not preclude a creator, but it does place limits on when he might have carried out his job! ? Stephen W. Hawking A Brief History of Time: From the Big Bang to Black Holes (1988), 8-9.
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Cell – Nucleus Organelles and Cytoplasm
cell organelles parts membranes endoplasmic reticulum golgi apparatus mitochondrion proteins phospholipids ribosomes lysosomes water cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.75bk.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Cell – Nucleus Organelles and Cytoplasm
cell organelles parts membranes endoplasmic reticulum golgi apparatus mitochondrion proteins phospholipids ribosomes lysosomes water cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.75bk.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
cell organelles parts membranes endoplasmic reticulum golgi apparatus mitochondrion proteins phospholipids ribosomes lysosomes water cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.75bk.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
cell organelles parts membranes endoplasmic reticulum golgi apparatus mitochondrion proteins phospholipids ribosomes lysosomes water cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.75bk.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Cell – Nucleus Organelles and Cytoplasm
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Cell – Nucleus Organelles and Cytoplasm
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Mitochondrion
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus mitochondrion cytology normal
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.61b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Mitochondrion
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus mitochondrion cytology normal
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.61b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus mitochondrion cytology normal
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.61b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus mitochondrion cytology normal
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.61b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Mitochondrion
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Mitochondrion
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Golgi Apparatus
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108274b13.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Golgi Apparatus
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108274b13.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108274b13.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
cell organelles parts membranes endoplasmic reticulum cytoplasm golgi apparatus
Copyright 2012 Courtesy Ashley Davidoff MD 108274b13.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Golgi Apparatus
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Golgi Apparatus
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Endoplasmic Reticulum
cell organelles parts membranes endoplasmic reticulum cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.68b02k.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Endoplasmic Reticulum
cell organelles parts membranes endoplasmic reticulum cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.68b02k.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
cell organelles parts membranes endoplasmic reticulum cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.68b02k.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
cell organelles parts membranes endoplasmic reticulum cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 108275b04.68b02k.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Endoplasmic Reticulum
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Endoplasmic Reticulum
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Nucleus
cell organelles parts membranes nucleus chromatin cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 82835pj.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Nucleus
cell organelles parts membranes nucleus chromatin cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 82835pj.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
cell organelles parts membranes nucleus chromatin cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 82835pj.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
cell organelles parts membranes nucleus chromatin cytoplasm
Copyright 2012 Courtesy Ashley Davidoff MD 82835pj.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Nucleus
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Nucleus
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Cholesterol Acts as a Padding between the Phospholipd Tails
Cholesterol Padding Other fats in the membrane Cholesterol molecules – prevent the phospholipid tails from sticking to each other cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc04.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Cholesterol Acts as a Padding between the Phospholipd Tails
Cholesterol Padding Other fats in the membrane Cholesterol molecules – prevent the phospholipid tails from sticking to each other cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc04.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Cholesterol Padding Other fats in the membrane Cholesterol molecules – prevent the phospholipid tails from sticking to each other cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc04.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Cholesterol Padding Other fats in the membrane Cholesterol molecules – prevent the phospholipid tails from sticking to each other cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc04.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Cholesterol Acts as a Padding between the Phospholipd Tails
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Cholesterol Acts as a Padding between the Phospholipd Tails
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Carbohydrates in the Membrane
carbohydrates Glycoproteins and Glycolipids cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc03.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Carbohydrates in the Membrane
carbohydrates Glycoproteins and Glycolipids cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc03.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
carbohydrates Glycoproteins and Glycolipids cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc03.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
carbohydrates Glycoproteins and Glycolipids cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc03.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Carbohydrates in the Membrane
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Carbohydrates in the Membrane
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Proteins in the Cell Membranes
Proteins Transmembrane protein (Globular Protein Channel Protein) Alpha helix Protein Surface protein (peripheral protein) Gated Channel cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Proteins in the Cell Membranes
Proteins Transmembrane protein (Globular Protein Channel Protein) Alpha helix Protein Surface protein (peripheral protein) Gated Channel cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Proteins Transmembrane protein (Globular Protein Channel Protein) Alpha helix Protein Surface protein (peripheral protein) Gated Channel cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Proteins Transmembrane protein (Globular Protein Channel Protein) Alpha helix Protein Surface protein (peripheral protein) Gated Channel cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10bc01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Proteins in the Cell Membranes
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Proteins in the Cell Membranes
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Two LAyers Hydrophobic on the Inside and Hydrophilic FAcing Our and In to the Cytoplasm
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Two LAyers Hydrophobic on the Inside and Hydrophilic FAcing Our and In to the Cytoplasm
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b02b10b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Two LAyers Hydrophobic on the Inside and Hydrophilic FAcing Our and In to the Cytoplasm
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Two LAyers Hydrophobic on the Inside and Hydrophilic FAcing Our and In to the Cytoplasm
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Two Layers Facing Each Other
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b03.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Two Layers Facing Each Other
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b03.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b03.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Two layers Allow for dual properties hydrophillic and hydrophobic cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j07b03.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Two Layers Facing Each Other
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Two Layers Facing Each Other
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Head and Two Tails of a Phospolipid Membrane
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
head = dark blue (alcohol) + light blue (phosphate) +green (glycerol
tail = yellow (2 long chain fatty acids)
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j05.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Head and Two Tails of a Phospolipid Membrane
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
head = dark blue (alcohol) + light blue (phosphate) +green (glycerol
tail = yellow (2 long chain fatty acids)
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j05.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 4
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
head = dark blue (alcohol) + light blue (phosphate) +green (glycerol
tail = yellow (2 long chain fatty acids)
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j05.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline big bang to biology
head = dark blue (alcohol) + light blue (phosphate) +green (glycerol
tail = yellow (2 long chain fatty acids)
Copyright 2012 Courtesy Ashley Davidoff MD 108250b08j05.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Head and Two Tails of a Phospolipid Membrane
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Head and Two Tails of a Phospolipid Membrane
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Chemical Components of Cell Membranes
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b07.84s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Chemical Components of Cell Membranes
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b07.84s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b07.84s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
cell membranes fats proteins double layer phospholipids hydrophobic hydrophilic alcohol phosphate glycerol fatty acids choline
Copyright 2012 Courtesy Ashley Davidoff MD 108250b07.84s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Chemical Components of Cell Membranes
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Chemical Components of Cell Membranes
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Hemoglobin
Hemoglobin consists mostly of proteins aka the 4 “globin” chains. The hemoglobin molecule is composed four globular protein subunits. There is an alpha chain (alpha 1 and alpha 2) and a beta chain (beta 1 and beta 2) All the proteins stick together These proteins, are made up of sequences of amino acids. Both proteins must be present to enable the hemoglobin to carry and release oxygen normally. Before birth, the beta protein is not present. Instead a gamma protein is present in the fetus but is replaced by the beta chain at birth. Each protein chain tightly associated with a non-protein heme group. A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic ring, known as a porphyrin. The iron ion, which is the site of oxygen and C02 binding In binding, oxygen temporarily oxidizes (Fe2+) to (Fe3+)
Copyright 2012 all rights reserved Courtesy Ashley Davidoff MD 88528b03c02.81s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Hemoglobin
Hemoglobin consists mostly of proteins aka the 4 “globin” chains. The hemoglobin molecule is composed four globular protein subunits. There is an alpha chain (alpha 1 and alpha 2) and a beta chain (beta 1 and beta 2) All the proteins stick together These proteins, are made up of sequences of amino acids. Both proteins must be present to enable the hemoglobin to carry and release oxygen normally. Before birth, the beta protein is not present. Instead a gamma protein is present in the fetus but is replaced by the beta chain at birth. Each protein chain tightly associated with a non-protein heme group. A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic ring, known as a porphyrin. The iron ion, which is the site of oxygen and C02 binding In binding, oxygen temporarily oxidizes (Fe2+) to (Fe3+)
Copyright 2012 all rights reserved Courtesy Ashley Davidoff MD 88528b03c02.81s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Hemoglobin consists mostly of proteins aka the 4 “globin” chains. The hemoglobin molecule is composed four globular protein subunits. There is an alpha chain (alpha 1 and alpha 2) and a beta chain (beta 1 and beta 2) All the proteins stick together These proteins, are made up of sequences of amino acids. Both proteins must be present to enable the hemoglobin to carry and release oxygen normally. Before birth, the beta protein is not present. Instead a gamma protein is present in the fetus but is replaced by the beta chain at birth. Each protein chain tightly associated with a non-protein heme group. A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic ring, known as a porphyrin. The iron ion, which is the site of oxygen and C02 binding In binding, oxygen temporarily oxidizes (Fe2+) to (Fe3+)
Copyright 2012 all rights reserved Courtesy Ashley Davidoff MD 88528b03c02.81s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Hemoglobin consists mostly of proteins aka the 4 “globin” chains. The hemoglobin molecule is composed four globular protein subunits. There is an alpha chain (alpha 1 and alpha 2) and a beta chain (beta 1 and beta 2) All the proteins stick together These proteins, are made up of sequences of amino acids. Both proteins must be present to enable the hemoglobin to carry and release oxygen normally. Before birth, the beta protein is not present. Instead a gamma protein is present in the fetus but is replaced by the beta chain at birth. Each protein chain tightly associated with a non-protein heme group. A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic ring, known as a porphyrin. The iron ion, which is the site of oxygen and C02 binding In binding, oxygen temporarily oxidizes (Fe2+) to (Fe3+)
Copyright 2012 all rights reserved Courtesy Ashley Davidoff MD 88528b03c02.81s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Hemoglobin
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Hemoglobin
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
78612pb01.58kb05L04.8s
DNA double helix uncoil transfer encryption messenger messenger RNA transcription RNA sugar ribose base protein cytosine guanine adenine tymine uracil
a)= DNA in spiral b) uncoling c) separating d) RNA match up e) RNA encoded f) base pairs in DNA and RNA
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L04.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
78612pb01.58kb05L04.8s
DNA double helix uncoil transfer encryption messenger messenger RNA transcription RNA sugar ribose base protein cytosine guanine adenine tymine uracil
a)= DNA in spiral b) uncoling c) separating d) RNA match up e) RNA encoded f) base pairs in DNA and RNA
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L04.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
DNA double helix uncoil transfer encryption messenger messenger RNA transcription RNA sugar ribose base protein cytosine guanine adenine tymine uracil
a)= DNA in spiral b) uncoling c) separating d) RNA match up e) RNA encoded f) base pairs in DNA and RNA
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L04.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
DNA double helix uncoil transfer encryption messenger messenger RNA transcription RNA sugar ribose base protein cytosine guanine adenine tymine uracil
a)= DNA in spiral b) uncoling c) separating d) RNA match up e) RNA encoded f) base pairs in DNA and RNA
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L04.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
78612pb01.58kb05L04.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
78612pb01.58kb05L04.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
78612pb01.58kb05L.8s
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
78612pb01.58kb05L.8s
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb05L.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
78612pb01.58kb05L.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
78612pb01.58kb05L.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
RNA Ribose is the Sugar
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L02.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
RNA Ribose is the Sugar
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L02.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L02.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
RNA Sugar ribose base protein cytosine guanine adenine uracil
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L02.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
RNA Ribose is the Sugar
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
RNA Ribose is the Sugar
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Double Helix
chromosome genes double helix DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.67kb06.82s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Double Helix
chromosome genes double helix DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.67kb06.82s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
chromosome genes double helix DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.67kb06.82s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
chromosome genes double helix DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.67kb06.82s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Double Helix
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Double Helix
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
78612pb01.58kb03b08.3k.81s
chromosomes genes double spiral DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb03b08.3k.81s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
78612pb01.58kb03b08.3k.81s
chromosomes genes double spiral DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb03b08.3k.81s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
chromosomes genes double spiral DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb03b08.3k.81s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
chromosomes genes double spiral DNA sugar base protein cytosine guanine adenine thymine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb03b08.3k.81s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
78612pb01.58kb03b08.3k.81s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
78612pb01.58kb03b08.3k.81s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
DNA sugar base protein cytosine guanine
DNA sugar base protein cytosine guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc02L.81s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
DNA sugar base protein cytosine guanine
DNA sugar base protein cytosine guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc02L.81s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => DNA sugar base protein cytosine guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc02L.81s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => DNA sugar base protein cytosine guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc02L.81s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
DNA sugar base protein cytosine guanine
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
DNA sugar base protein cytosine guanine
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Bonnding of Two BAses Thymine and Cytosine
the bases will bond via hydrogen bonds adenine only to thymine and cytosine only to guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc01.83s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Bonnding of Two BAses Thymine and Cytosine
the bases will bond via hydrogen bonds adenine only to thymine and cytosine only to guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc01.83s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
the bases will bond via hydrogen bonds adenine only to thymine and cytosine only to guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc01.83s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
the bases will bond via hydrogen bonds adenine only to thymine and cytosine only to guanine
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01b02bc01.83s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Bonnding of Two BAses Thymine and Cytosine
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Bonnding of Two BAses Thymine and Cytosine
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
DNA – Sugar is Dexyribose
DNA A base is a a substance that can accept hydrogen ions (protons) or more generally, donate electron pairs. The base in the nucleotide is a nitrogen containing base = nitrogenous base
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
DNA – Sugar is Dexyribose
DNA A base is a a substance that can accept hydrogen ions (protons) or more generally, donate electron pairs. The base in the nucleotide is a nitrogen containing base = nitrogenous base
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
DNA A base is a a substance that can accept hydrogen ions (protons) or more generally, donate electron pairs. The base in the nucleotide is a nitrogen containing base = nitrogenous base
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
DNA A base is a a substance that can accept hydrogen ions (protons) or more generally, donate electron pairs. The base in the nucleotide is a nitrogen containing base = nitrogenous base
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
DNA – Sugar is Dexyribose
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
DNA – Sugar is Dexyribose
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
phosphate sugar and a base = nucleotide
phosphate sugar and a base = nucleotide
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
phosphate sugar and a base = nucleotide
phosphate sugar and a base = nucleotide
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
phosphate sugar and a base = nucleotide
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
phosphate sugar and a base = nucleotide
Copyright 2012 Courtesy Ashley Davidoff MD 78612pb01.58kb02L.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
phosphate sugar and a base = nucleotide
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
phosphate sugar and a base = nucleotide
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Complex Shapes of Protein Molecules
complex shape 3D protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.84.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Complex Shapes of Protein Molecules
complex shape 3D protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.84.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
complex shape 3D protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.84.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
complex shape 3D protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.84.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Complex Shapes of Protein Molecules
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Complex Shapes of Protein Molecules
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
108246b04b09b.8s
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
108246b04b09b.8s
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
108246b04b09b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
108246b04b09b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
3D protein Shapes in 2D
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.8.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
3D protein Shapes in 2D
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.8.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.8.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
protein big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 108246b04b09.8.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
3D protein Shapes in 2D
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
3D protein Shapes in 2D
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
alpha helix and beta pleated sheet protein
alpha helix and beta pleated sheet protein
Copyright 2012 Courtesy Ashley Davidoff MD 108247b01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
alpha helix and beta pleated sheet protein
alpha helix and beta pleated sheet protein
Copyright 2012 Courtesy Ashley Davidoff MD 108247b01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
alpha helix and beta pleated sheet protein
Copyright 2012 Courtesy Ashley Davidoff MD 108247b01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
alpha helix and beta pleated sheet protein
Copyright 2012 Courtesy Ashley Davidoff MD 108247b01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
alpha helix and beta pleated sheet protein
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
alpha helix and beta pleated sheet protein
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Protein with Specific Shape
The Units Link The physical shape of the proteins is their most unique characteristics aspect that allows functional specificity http://www.youtube.com/watch?v=lijQ3a8yUYQ
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.45.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Protein with Specific Shape
The Units Link The physical shape of the proteins is their most unique characteristics aspect that allows functional specificity http://www.youtube.com/watch?v=lijQ3a8yUYQ
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.45.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Units Link The physical shape of the proteins is their most unique characteristics aspect that allows functional specificity http://www.youtube.com/watch?v=lijQ3a8yUYQ
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.45.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Units Link The physical shape of the proteins is their most unique characteristics aspect that allows functional specificity http://www.youtube.com/watch?v=lijQ3a8yUYQ
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.45.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Protein with Specific Shape
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Protein with Specific Shape
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The 20 Amino Acids
The 20 amino acids
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c13iL.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The 20 Amino Acids
The 20 amino acids
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c13iL.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The 20 amino acids
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c13iL.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The 20 amino acids
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c13iL.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The 20 Amino Acids
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The 20 Amino Acids
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Amino Acid – building block of protein
Alpha carbon the connector with the “R” representing something else eg hydrogen or CH3 big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a03.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Amino Acid – building block of protein
Alpha carbon the connector with the “R” representing something else eg hydrogen or CH3 big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a03.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Alpha carbon the connector with the “R” representing something else eg hydrogen or CH3 big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a03.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Alpha carbon the connector with the “R” representing something else eg hydrogen or CH3 big bang to biology
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a03.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Amino Acid – building block of protein
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Amino Acid – building block of protein
)
https://beta.thecommonvein.net/wp-content/uploads/2023/09/87541pcd.01b01c16a03.8s.jpg
http://www.thecommonvein.net/media/87541pcd.01b01c16a03.8s_1.jpg
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Amine NH3 proteins
Amine NH3 proteins
Alpha Carbon – The Link
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a02b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Amine NH3 proteins
Amine NH3 proteins
Alpha Carbon – The Link
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a02b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Amine NH3 proteins
Alpha Carbon – The Link
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a02b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Amine NH3 proteins
Alpha Carbon – The Link
Copyright 2012 Courtesy Ashley Davidoff MD 87541pcd.01b01c16a02b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Amine NH3 proteins
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Amine NH3 proteins
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Triglycerides
The bond or link of the OH groups on the glycerol side links with the carboxyl group (carboxylic acid) OH + COOH
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf08b01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Triglycerides
The bond or link of the OH groups on the glycerol side links with the carboxyl group (carboxylic acid) OH + COOH
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf08b01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The bond or link of the OH groups on the glycerol side links with the carboxyl group (carboxylic acid) OH + COOH
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf08b01.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The bond or link of the OH groups on the glycerol side links with the carboxyl group (carboxylic acid) OH + COOH
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf08b01.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Triglycerides
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Triglycerides
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Glycerol
Glycerol The OH groups – hydroxy functional groups place glcerol into the alcohol groups of chemicals “ol” denotes its alcohol nature diagram http://www.green-planet-solar-energy.com/what-is-glycerin.html
Copyright 2012 Courtesy Ashley Davidoff MD 70983g01.8S
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Glycerol
Glycerol The OH groups – hydroxy functional groups place glcerol into the alcohol groups of chemicals “ol” denotes its alcohol nature diagram http://www.green-planet-solar-energy.com/what-is-glycerin.html
Copyright 2012 Courtesy Ashley Davidoff MD 70983g01.8S
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Glycerol The OH groups – hydroxy functional groups place glcerol into the alcohol groups of chemicals “ol” denotes its alcohol nature diagram http://www.green-planet-solar-energy.com/what-is-glycerin.html
Copyright 2012 Courtesy Ashley Davidoff MD 70983g01.8S
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Glycerol The OH groups – hydroxy functional groups place glcerol into the alcohol groups of chemicals “ol” denotes its alcohol nature diagram http://www.green-planet-solar-energy.com/what-is-glycerin.html
Copyright 2012 Courtesy Ashley Davidoff MD 70983g01.8S
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Glycerol
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Glycerol
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
70983.4kf09L.1ks01Ls
The unique linking and organization of the carbons hydrogens and oxygen creates unique products or metabolites, This linking is not indiscriminate. There are rules that the units have to abide by in order that organization can take place. An obvious rule in this instance is that the carbon can only mintain 4 bonds A double bond takes up two of the availablke 4 . Since each of the bonded carbons use up 2 bonds with the double bond, and are connected to another carbon on each side and threfore they only have room for one hydrogen each.
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks01Ls
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
70983.4kf09L.1ks01Ls
The unique linking and organization of the carbons hydrogens and oxygen creates unique products or metabolites, This linking is not indiscriminate. There are rules that the units have to abide by in order that organization can take place. An obvious rule in this instance is that the carbon can only mintain 4 bonds A double bond takes up two of the availablke 4 . Since each of the bonded carbons use up 2 bonds with the double bond, and are connected to another carbon on each side and threfore they only have room for one hydrogen each.
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks01Ls
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The unique linking and organization of the carbons hydrogens and oxygen creates unique products or metabolites, This linking is not indiscriminate. There are rules that the units have to abide by in order that organization can take place. An obvious rule in this instance is that the carbon can only mintain 4 bonds A double bond takes up two of the availablke 4 . Since each of the bonded carbons use up 2 bonds with the double bond, and are connected to another carbon on each side and threfore they only have room for one hydrogen each.
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks01Ls
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The unique linking and organization of the carbons hydrogens and oxygen creates unique products or metabolites, This linking is not indiscriminate. There are rules that the units have to abide by in order that organization can take place. An obvious rule in this instance is that the carbon can only mintain 4 bonds A double bond takes up two of the availablke 4 . Since each of the bonded carbons use up 2 bonds with the double bond, and are connected to another carbon on each side and threfore they only have room for one hydrogen each.
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks01Ls
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
70983.4kf09L.1ks01Ls
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
70983.4kf09L.1ks01Ls
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Fatty Acid
fatty acid
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Fatty Acid
fatty acid
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
fatty acid
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
fatty acid
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf09L.1ks
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Fatty Acid
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Fatty Acid
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Fats A Combination of C H and O
Fats A Combination of C H and O
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.82s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Fats A Combination of C H and O
Fats A Combination of C H and O
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.82s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Fats A Combination of C H and O
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.82s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Fats A Combination of C H and O
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.82s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Fats A Combination of C H and O
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Fats A Combination of C H and O
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Carbohydrate Monosaccharride Glucose
Carbohydrate monosaccharide glucose
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf02.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Carbohydrate Monosaccharride Glucose
Carbohydrate monosaccharide glucose
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf02.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Carbohydrate monosaccharide glucose
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf02.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Carbohydrate monosaccharide glucose
Copyright 2012 Courtesy Ashley Davidoff MD 70983.4kf02.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Carbohydrate Monosaccharride Glucose
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Carbohydrate Monosaccharride Glucose
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Carbohydrates
C:2H:O
Carbohydrates Carbohydrates (hydrates of carbon) are organic compounds that usually contain carbon hydrogen and oxygen in the ratios: 1 Carbon: 2 Hydrogens: 1 Oxygen.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.83s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Carbohydrates
C:2H:O
Carbohydrates Carbohydrates (hydrates of carbon) are organic compounds that usually contain carbon hydrogen and oxygen in the ratios: 1 Carbon: 2 Hydrogens: 1 Oxygen.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.83s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Carbohydrates Carbohydrates (hydrates of carbon) are organic compounds that usually contain carbon hydrogen and oxygen in the ratios: 1 Carbon: 2 Hydrogens: 1 Oxygen.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.83s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Carbohydrates Carbohydrates (hydrates of carbon) are organic compounds that usually contain carbon hydrogen and oxygen in the ratios: 1 Carbon: 2 Hydrogens: 1 Oxygen.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b25.83s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Carbohydrates
C:2H:O
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Carbohydrates
C:2H:O
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Hydrogen Oxygen Carbon Nitrogen
The Most Common Building Blocks of Biological Materials
The Most Common Building Blocks of Biological Materials
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.81
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Hydrogen Oxygen Carbon Nitrogen
The Most Common Building Blocks of Biological Materials
The Most Common Building Blocks of Biological Materials
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.81
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Most Common Building Blocks of Biological Materials
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.81
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Most Common Building Blocks of Biological Materials
Copyright 2012 Courtesy Ashley Davidoff MD 108485p.81
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Hydrogen Oxygen Carbon Nitrogen
The Most Common Building Blocks of Biological Materials
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Hydrogen Oxygen Carbon Nitrogen
The Most Common Building Blocks of Biological Materials
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Two Hydrogens and One Oxygen
Water Molecule
The Molecule Two Hydrogens and an Oxygen Water Davidoff art
Copyright 2012 Courtesy Ashley Davidoff MD 13451b23.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Two Hydrogens and One Oxygen
Water Molecule
The Molecule Two Hydrogens and an Oxygen Water Davidoff art
Copyright 2012 Courtesy Ashley Davidoff MD 13451b23.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Molecule Two Hydrogens and an Oxygen Water Davidoff art
Copyright 2012 Courtesy Ashley Davidoff MD 13451b23.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Molecule Two Hydrogens and an Oxygen Water Davidoff art
Copyright 2012 Courtesy Ashley Davidoff MD 13451b23.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Two Hydrogens and One Oxygen
Water Molecule
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Two Hydrogens and One Oxygen
Water Molecule
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Elements
The Elements Now up to 118 of them Periodic Table in the Round 108485pb20.8s
Copyright 2012 Courtesy Ashley Davidoff MD 108485pb20.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Elements
The Elements Now up to 118 of them Periodic Table in the Round 108485pb20.8s
Copyright 2012 Courtesy Ashley Davidoff MD 108485pb20.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Elements Now up to 118 of them Periodic Table in the Round 108485pb20.8s
Copyright 2012 Courtesy Ashley Davidoff MD 108485pb20.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Elements Now up to 118 of them Periodic Table in the Round 108485pb20.8s
Copyright 2012 Courtesy Ashley Davidoff MD 108485pb20.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Elements
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Elements
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Formation of an Element
units person body atom solar system universe TCV Davidoff art concept
Copyright 2012 Courtesy Ashley Davidoff MD 45827b10.800
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Formation of an Element
units person body atom solar system universe TCV Davidoff art concept
Copyright 2012 Courtesy Ashley Davidoff MD 45827b10.800
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
units person body atom solar system universe TCV Davidoff art concept
Copyright 2012 Courtesy Ashley Davidoff MD 45827b10.800
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
units person body atom solar system universe TCV Davidoff art concept
Copyright 2012 Courtesy Ashley Davidoff MD 45827b10.800
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Formation of an Element
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Formation of an Element
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Atom – The Powerful and Indivisible
This atom has 56 electrons in 6 orbits It is called barium
In this diagram of barium we note the electrons spinning around the nucleus Barium has 56 electrons, 56 protons and 81 neutrons giving it an atomic weight of 56+81 = 137. The electrons atre distributed in the orbits in the following manner 2,8,18,18,8,2.
Copyright 2012 Courtesy Ashley Davidoff MD 45824b13bd10.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Atom – The Powerful and Indivisible
This atom has 56 electrons in 6 orbits It is called barium
In this diagram of barium we note the electrons spinning around the nucleus Barium has 56 electrons, 56 protons and 81 neutrons giving it an atomic weight of 56+81 = 137. The electrons atre distributed in the orbits in the following manner 2,8,18,18,8,2.
Copyright 2012 Courtesy Ashley Davidoff MD 45824b13bd10.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
In this diagram of barium we note the electrons spinning around the nucleus Barium has 56 electrons, 56 protons and 81 neutrons giving it an atomic weight of 56+81 = 137. The electrons atre distributed in the orbits in the following manner 2,8,18,18,8,2.
Copyright 2012 Courtesy Ashley Davidoff MD 45824b13bd10.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
In this diagram of barium we note the electrons spinning around the nucleus Barium has 56 electrons, 56 protons and 81 neutrons giving it an atomic weight of 56+81 = 137. The electrons atre distributed in the orbits in the following manner 2,8,18,18,8,2.
Copyright 2012 Courtesy Ashley Davidoff MD 45824b13bd10.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Atom – The Powerful and Indivisible
This atom has 56 electrons in 6 orbits It is called barium
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Atom – The Powerful and Indivisible
This atom has 56 electrons in 6 orbits It is called barium
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Doubling Up the Forces – Helium
When two hydrogen atoms combined in a given environment, at a moment in time and space, helium the second lightest and second most abundant element in the universe is produced.
Copyright 2012 Courtesy Ashley Davidoff MD 84154c03.800b01
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Doubling Up the Forces – Helium
When two hydrogen atoms combined in a given environment, at a moment in time and space, helium the second lightest and second most abundant element in the universe is produced.
Copyright 2012 Courtesy Ashley Davidoff MD 84154c03.800b01
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
When two hydrogen atoms combined in a given environment, at a moment in time and space, helium the second lightest and second most abundant element in the universe is produced.
Copyright 2012 Courtesy Ashley Davidoff MD 84154c03.800b01
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
When two hydrogen atoms combined in a given environment, at a moment in time and space, helium the second lightest and second most abundant element in the universe is produced.
Copyright 2012 Courtesy Ashley Davidoff MD 84154c03.800b01
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Doubling Up the Forces – Helium
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Doubling Up the Forces – Helium
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Birth of the Proton from the Womb of the Hot Hot Hot Universe
Hydrogen, as simplest and lightest atom, remains the most abundant atom in the universe, and contributes to 90% of all the atoms of the universe.
Courtesy Ashley Davidoff MD 102976pb04b10b06b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Birth of the Proton from the Womb of the Hot Hot Hot Universe
Hydrogen, as simplest and lightest atom, remains the most abundant atom in the universe, and contributes to 90% of all the atoms of the universe.
Courtesy Ashley Davidoff MD 102976pb04b10b06b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Hydrogen, as simplest and lightest atom, remains the most abundant atom in the universe, and contributes to 90% of all the atoms of the universe.
Courtesy Ashley Davidoff MD 102976pb04b10b06b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Hydrogen, as simplest and lightest atom, remains the most abundant atom in the universe, and contributes to 90% of all the atoms of the universe.
Courtesy Ashley Davidoff MD 102976pb04b10b06b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Birth of the Proton from the Womb of the Hot Hot Hot Universe
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Birth of the Proton from the Womb of the Hot Hot Hot Universe
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Hydrogen Atom Spinning Precessing Magnetic Field
Mass, Motion, Space, Time and Electromagnetic Force
The spinning hydrogen atom introduces us to the elements of mass, motion, space, time, and electromagnetic forces.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b22.8.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Hydrogen Atom Spinning Precessing Magnetic Field
Mass, Motion, Space, Time and Electromagnetic Force
The spinning hydrogen atom introduces us to the elements of mass, motion, space, time, and electromagnetic forces.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b22.8.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The spinning hydrogen atom introduces us to the elements of mass, motion, space, time, and electromagnetic forces.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b22.8.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The spinning hydrogen atom introduces us to the elements of mass, motion, space, time, and electromagnetic forces.
Copyright 2012 Courtesy Ashley Davidoff MD 13451b22.8.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Hydrogen Atom Spinning Precessing Magnetic Field
Mass, Motion, Space, Time and Electromagnetic Force
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Hydrogen Atom Spinning Precessing Magnetic Field
Mass, Motion, Space, Time and Electromagnetic Force
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Formation of the Hydrogen Atom
After the big bang one of the first interactions between the electrons and protons was in the formation of the hydrogen atom An electron (unit 1) interacted with a proton (unit 2) in the heated environment in the space of the universe, at a moment in time, and a new and unique unit 3, called the hydrogen atom was formed. because the interaction resulted in a spinning charge an electromagnetic field was set up and the atom also spun in space in a unique way that was not characteristic of either the proton or the electron.
Copyright 2012 Courtesy Ashley Davidoff MD 102976pb03b04.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Formation of the Hydrogen Atom
After the big bang one of the first interactions between the electrons and protons was in the formation of the hydrogen atom An electron (unit 1) interacted with a proton (unit 2) in the heated environment in the space of the universe, at a moment in time, and a new and unique unit 3, called the hydrogen atom was formed. because the interaction resulted in a spinning charge an electromagnetic field was set up and the atom also spun in space in a unique way that was not characteristic of either the proton or the electron.
Copyright 2012 Courtesy Ashley Davidoff MD 102976pb03b04.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
After the big bang one of the first interactions between the electrons and protons was in the formation of the hydrogen atom An electron (unit 1) interacted with a proton (unit 2) in the heated environment in the space of the universe, at a moment in time, and a new and unique unit 3, called the hydrogen atom was formed. because the interaction resulted in a spinning charge an electromagnetic field was set up and the atom also spun in space in a unique way that was not characteristic of either the proton or the electron.
Copyright 2012 Courtesy Ashley Davidoff MD 102976pb03b04.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
After the big bang one of the first interactions between the electrons and protons was in the formation of the hydrogen atom An electron (unit 1) interacted with a proton (unit 2) in the heated environment in the space of the universe, at a moment in time, and a new and unique unit 3, called the hydrogen atom was formed. because the interaction resulted in a spinning charge an electromagnetic field was set up and the atom also spun in space in a unique way that was not characteristic of either the proton or the electron.
Copyright 2012 Courtesy Ashley Davidoff MD 102976pb03b04.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Formation of the Hydrogen Atom
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Formation of the Hydrogen Atom
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Primal Electromagnetic Forces
Positive and Negative
The primal opposites, attracted each other’ like nothing else, forming an unavoidable union, while retaining their individuality and going for the most part their separate ways, but remaining bonded. The first and most abundant union and the building block of all to follow is the hydrogen atom consisting of one electron and one proton.
Copyright 2012 Courtesy Ashley Davidoff MD 84154b04.698
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Primal Electromagnetic Forces
Positive and Negative
The primal opposites, attracted each other’ like nothing else, forming an unavoidable union, while retaining their individuality and going for the most part their separate ways, but remaining bonded. The first and most abundant union and the building block of all to follow is the hydrogen atom consisting of one electron and one proton.
Copyright 2012 Courtesy Ashley Davidoff MD 84154b04.698
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The primal opposites, attracted each other’ like nothing else, forming an unavoidable union, while retaining their individuality and going for the most part their separate ways, but remaining bonded. The first and most abundant union and the building block of all to follow is the hydrogen atom consisting of one electron and one proton.
Copyright 2012 Courtesy Ashley Davidoff MD 84154b04.698
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The primal opposites, attracted each other’ like nothing else, forming an unavoidable union, while retaining their individuality and going for the most part their separate ways, but remaining bonded. The first and most abundant union and the building block of all to follow is the hydrogen atom consisting of one electron and one proton.
Copyright 2012 Courtesy Ashley Davidoff MD 84154b04.698
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Primal Electromagnetic Forces
Positive and Negative
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Primal Electromagnetic Forces
Positive and Negative
)
https://beta.thecommonvein.net/wp-content/uploads/2023/03/84154b04.698.jpg
http://www.thecommonvein.net/media/84154b04.698_1.jpg
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Birth of Protons, Electrons, and Neutrons
When the universe started to cool the electrons, protons and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff 102976pb04b06b01b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Birth of Protons, Electrons, and Neutrons
When the universe started to cool the electrons, protons and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff 102976pb04b06b01b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
When the universe started to cool the electrons, protons and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff 102976pb04b06b01b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
When the universe started to cool the electrons, protons and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff 102976pb04b06b01b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
The Birth of Protons, Electrons, and Neutrons
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
The Birth of Protons, Electrons, and Neutrons
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Big Bang
In the beginning – about 13.7 billion years ago there was a big bang and the universe was formed. It was very hot but when it started to cool, electrons, protons, and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff MD 102976p.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Big Bang
In the beginning – about 13.7 billion years ago there was a big bang and the universe was formed. It was very hot but when it started to cool, electrons, protons, and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff MD 102976p.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
In the beginning – about 13.7 billion years ago there was a big bang and the universe was formed. It was very hot but when it started to cool, electrons, protons, and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff MD 102976p.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
In the beginning – about 13.7 billion years ago there was a big bang and the universe was formed. It was very hot but when it started to cool, electrons, protons, and neutrons were born.
Copyright 2012 Courtesy Ashley Davidoff MD 102976p.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Big Bang
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Big Bang
)