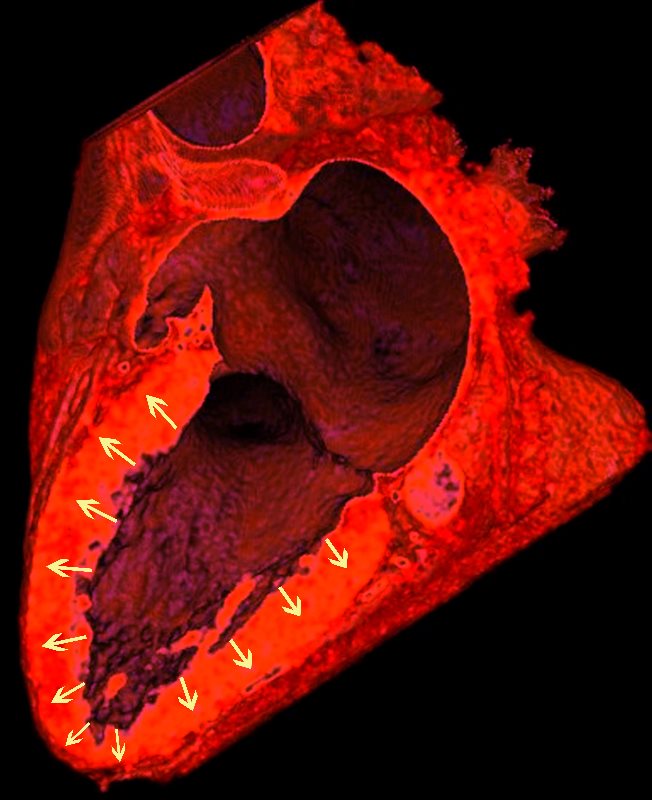
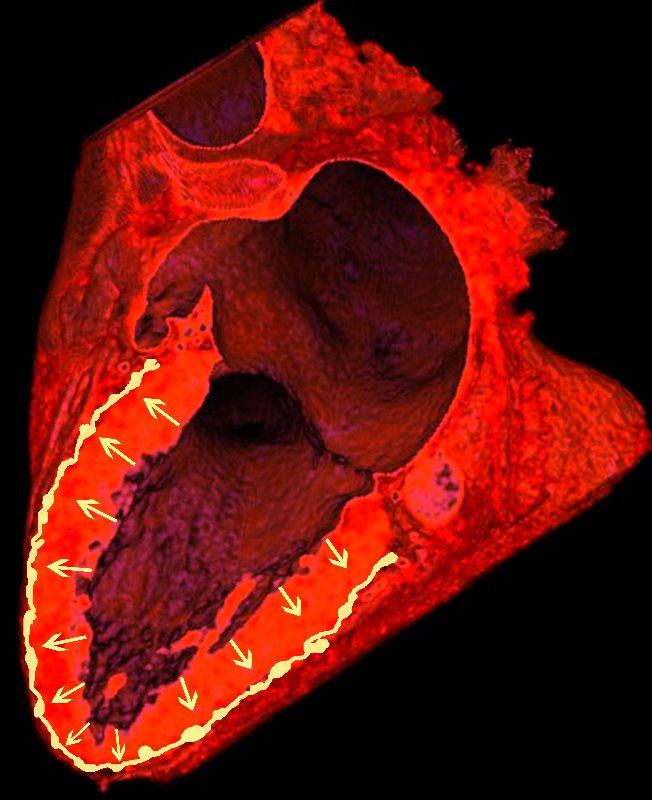
In a Nutshell
As in the lungs the the granulomatous nodules follow the lymphatics
- MRI LGE
- Heart patchy mid multifocal and epi
- located predominantly in the subepicardial areas,
- Also myocardial, and least common subendocardial
- most characteristic free wall and basal septum (conduction)
Cardiac sarcoidosis (CS)detected in 20-30% of patients with sarcoidosis (up to 55% in Japanese population) and more than 20% of the cases of sarcoidosis are clinically silent. Complete heart block, bundle branch block, ventricular tachycardia (VT), congestive heart failure (HF), and sudden death are common presentations in CS
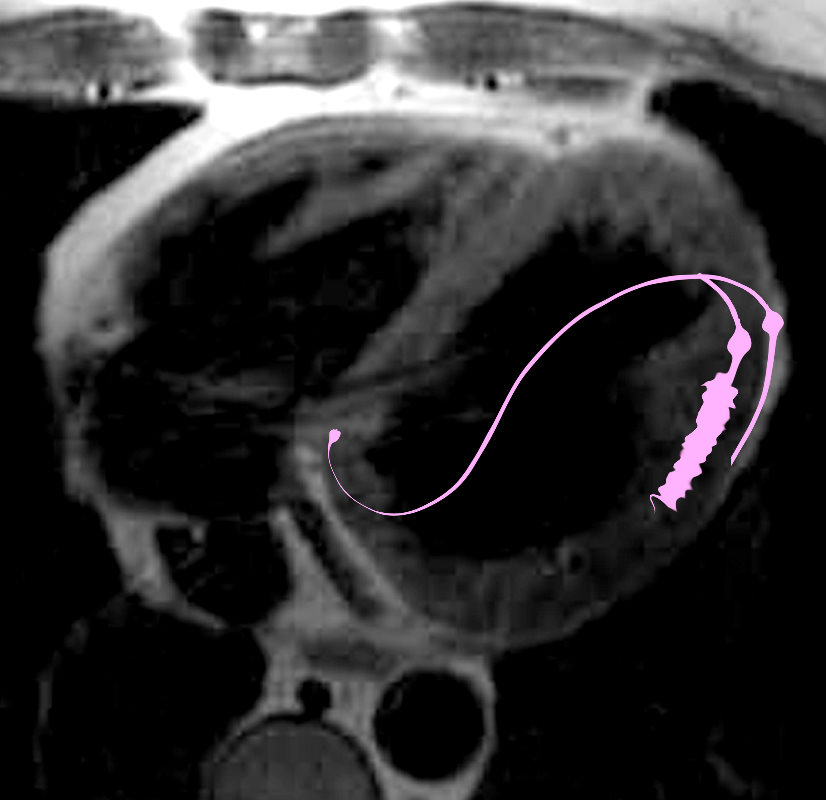
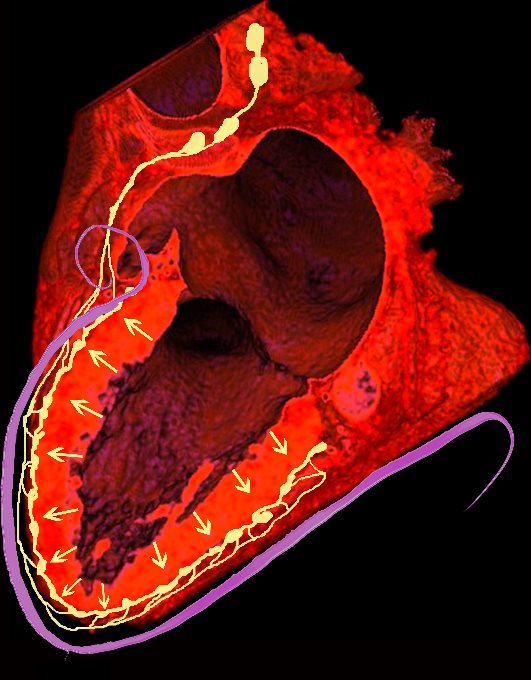
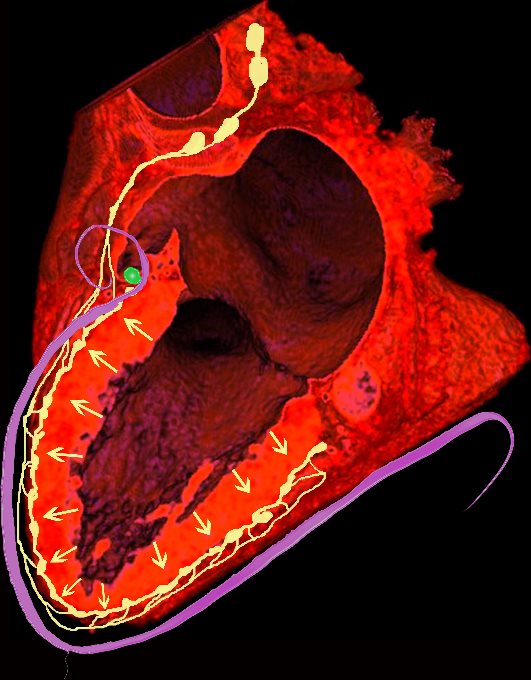
NORMAL LYMPHATIC DRAINAGE
Lymphatics run from the subendocardium through the myocardium to the subendocardial layer where they accumulate in subendocardial plexuses
The subendocardial plexuses drain into lymphatics which accompany the coronary arteries which then drain into mediastinal nodes
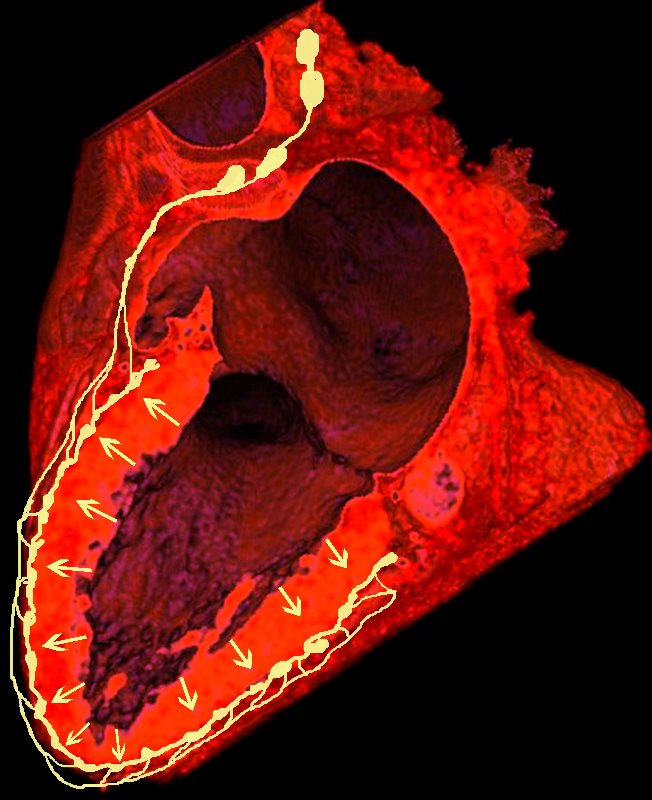
The lymph drains from the subendocardium through the myocardium where it collects in subendocardial plexuses and then into lymphatic vessels which travel along with the LAD
Ashley Davidoff MD
SARCOIDOSIS
25-30% cardiac involvement
No portion of the heart is immune to infiltration by sarcoid granulomas. The granulomas may involve the pericardium, myocardium, and endocardium, but of the three, the myocardium is, by far, the one most frequently involved. (sarcoid = meat – so myocardium most commonly involved)
- Structures in the CV system affected by sarcoidosis
- LV
- RV
- Conduction System
- Pericardium
- Pulmonary Artery
LV
The “S” of Sarcoidosis
The hallmark of cardiac sarcoidosis is LGE involving subepicardial regions of lateral free wall as well as medial basal septum but also mid myocardial with linear patchy and nodular forms.
Biventricular involvement is common
Ashley Davidoff MD
The most common involvement observed on MRI is the subepicardial layer
Ashley Davidoff MD
The pattern of LGE is typically patchy, mid-myocardial or epicardial and in a non-coronary distribution. LGE can be seen in both active disease (due to inflammation) and in scar.
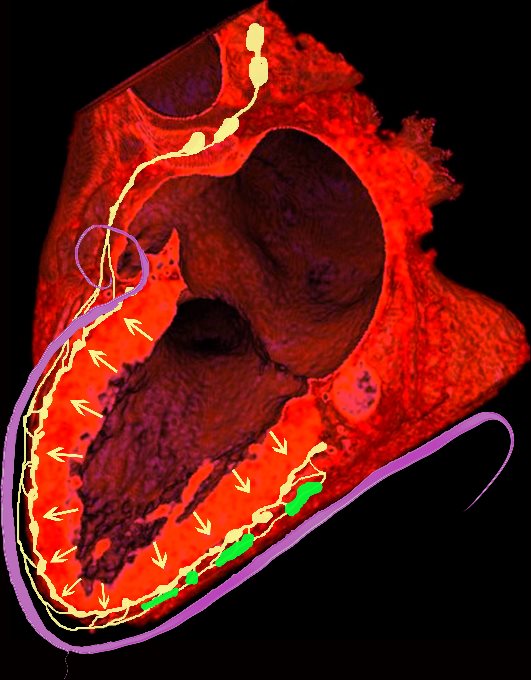
The hallmark of cardiac sarcoidosis is LGE involving subepicardial regions of lateral free wall as well as medial basal septum but also mid myocardial with linear patchy and nodular forms.
Biventricular involvement is common
Ashley Davidoff MD
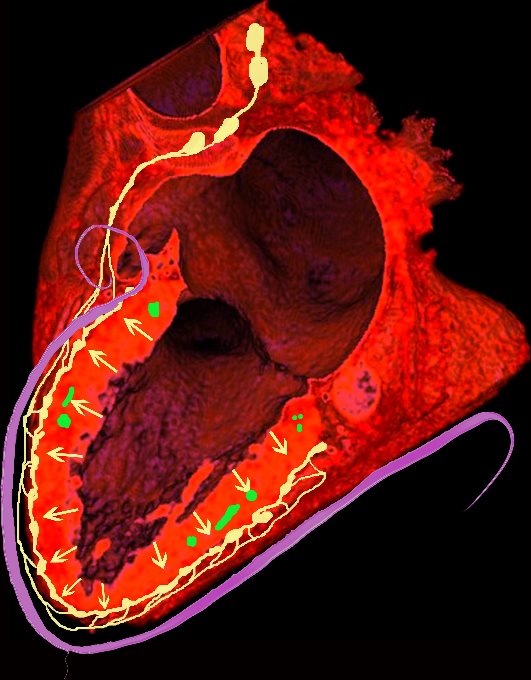
Ashley Davidoff MD
Clinical Examples
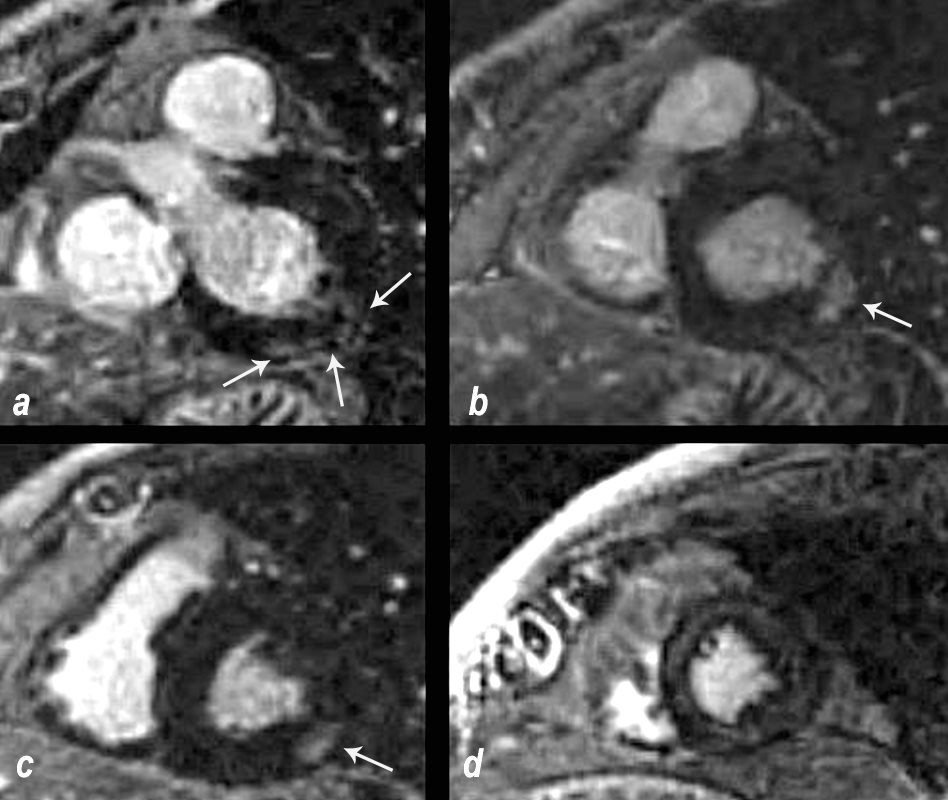
69 year old male presented with history of cardiomyopathy and atrial fibrillation The findings on MRI are highly suggestive of sarcoidosis. There are multicentric foci of LGE in linear and nodular form in the mid myocardial and subepicardial layers and likely in the pericardium and myocardium of the right ventricle.
There was associated global hypokinesis of the LV with an EF of 40%, and increase in the LV mass of 120gms/ sq m
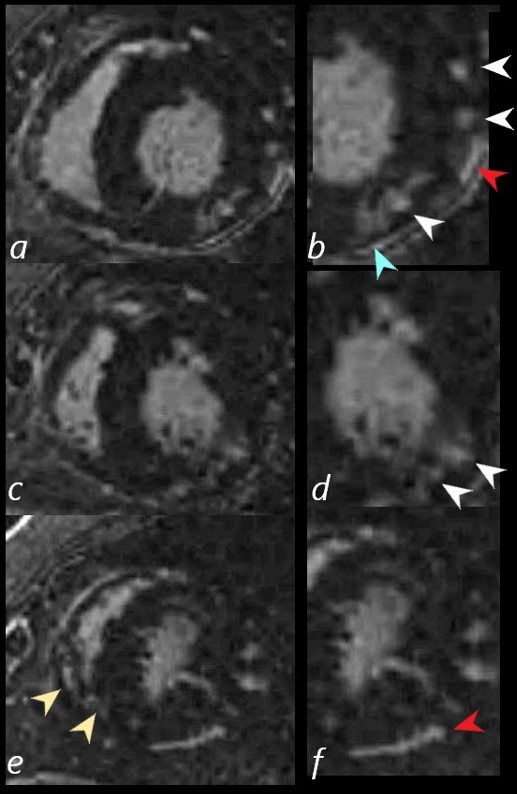
In this series images on the right (b,,d,,f) are magnified views of images on the left (a,c,e)
There are multicentric foci of LGE nodular form in the mid myocardial region (white arrowheads) linear LGE in the subepicardial layers (red arrowheads, b and f) in the pericardium (teal blue arrowhead) and myocardium of the right ventricle (yellow arrowhead e).
Ashley Davidoff MD
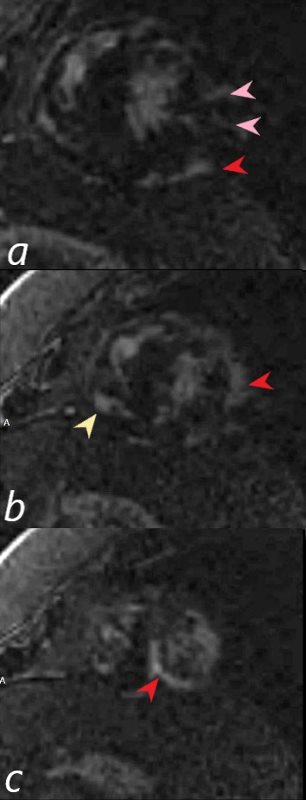
There are multicentric foci of LGE in this short axis view taken closer to the apex with linear form in the mid myocardial region (pink arrowheads) and linear and nodular changes of LGE in the subepicardial layers (red arrowheads, a,b,c) Subepicardial changes are also notes in the RV in a (not marked) Ashley Davidoff MD
69 year old male presented with history of cardiomyopathy and atrial fibrillation
In this series, the long axis 4 chambered view reveals intense continuous mid myocardial LGE
Ashley Davidoff MD
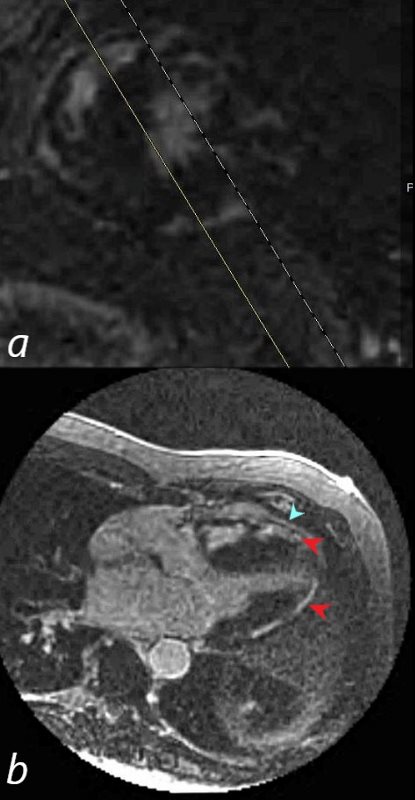
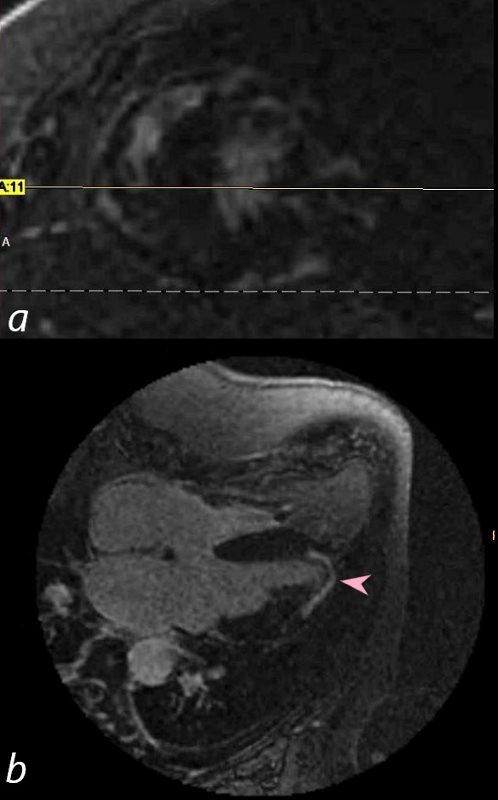
69-year-old male presented with history of cardiomyopathy and atrial fibrillation
In this series the long axis 3 chambered view reveals intense continuous sub epicardial disease (red arrowheads) and pericardial LE (teal blue arrowhead)
Ashley Davidoff MD
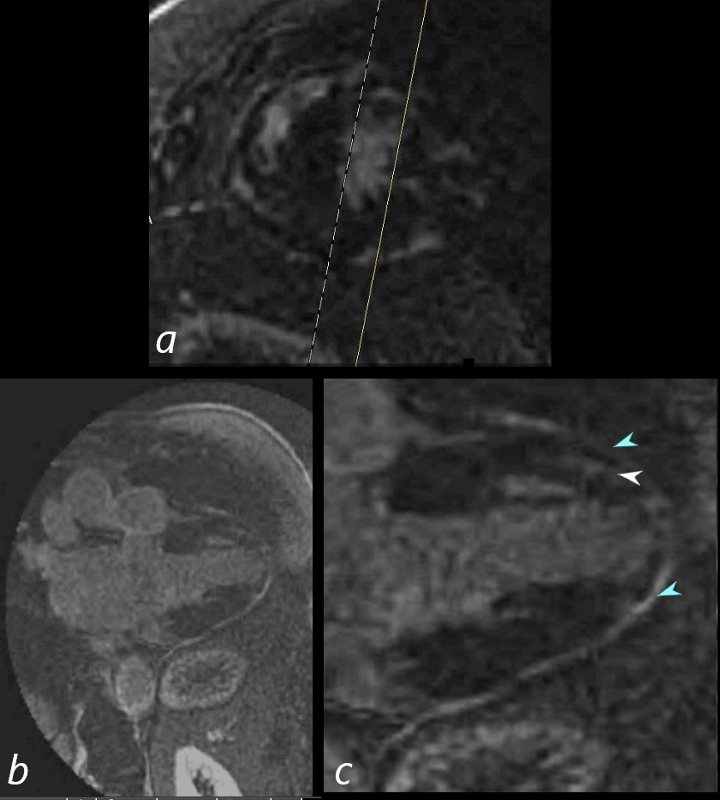
69-year-old male presented with history of cardiomyopathy and atrial fibrillation
In this series, the long axis 2 chambered view (c is magnified view of b) reveals intense continuous pericardial disease (teal blue arrowheads) and mid myocardial linear-nodular LGE (white arrowhead)
Ashley Davidoff MD
Ashley Davidoff MD
34-year-old male who was diagnosed with pulmonary sarcoidosis 1 year before, presents with progressive dyspnea over 6 months, previously able to run 5 miles per day and currently limited to walking short distances. Clinical evaluation revealed that he was in complete heart block with a heart rate of 35 /min. EKG showed a rate of 40 with AV dissociation with alternating junctional and ventricular escape rhythms.
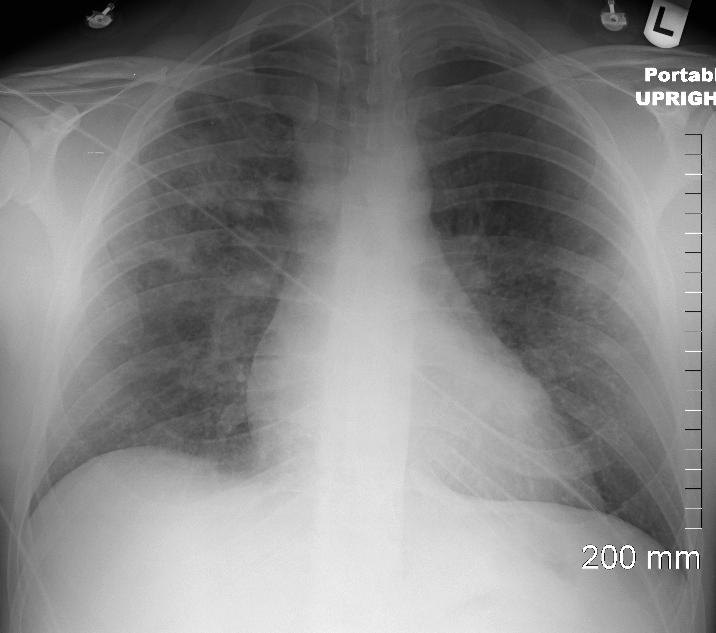
CXR showed upper lobe predominance interstitial changes, slightly more prominent on the right side. There was evidence of CHF with enlargement of the LA (widening of the carinal angle) and cephalization of the vessels
An echocardiogram was normal with normal ejection fraction.
MRI revealed normal cardiac function, mild left atrial enlargement, and diffuse almost circumferential subendocardial enhancement of the LV, most prominent in the lateral wall. There was also a linear focus in the mid myocardium in the anterior region medially near the septum
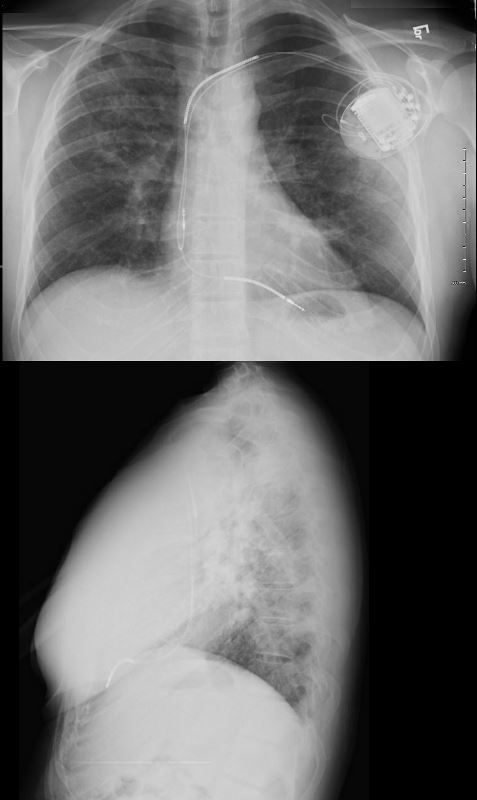
A dual lead pacemaker was placed and follow up CXR showed improved CHF an persistent interstitial findings dominant in the upper lobes consistent with sarcoidosis.
Follow up CXR 7 years later showed a normal CXR. The pacemaker had been removed and the interstitial process had resolved
Ashley Davidoff MD
34-year-old male who was diagnosed with pulmonary sarcoidosis 1 year before, presents with progressive dyspnea over 6 months, previously able to run 5 miles per day and currently limited to walking short distances. Clinical evaluation revealed that he was in complete heart block with a heart rate of 35 /min. EKG showed a rate of 40 with AV dissociation with alternating junctional and ventricular escape rhythms.
CXR showed upper lobe predominance interstitial changes, slightly more prominent on the right side. There was evidence of CHF with enlargement of the LA (widening of the carinal angle) and cephalization of the vessels
An echocardiogram was normal with normal ejection fraction.
MRI revealed normal cardiac function, mild left atrial enlargement, and diffuse almost circumferential subendocardial enhancement of the LV, most prominent in the lateral wall. There was also a linear focus in the mid myocardium in the anterior region medially near the septum
A dual lead pacemaker was placed and follow up CXR showed improved CHF an persistent interstitial findings dominant in the upper lobes consistent with sarcoidosis.
Follow up CXR 7 years later showed a normal CXR. The pacemaker had been removed and the interstitial process had resolved
Ashley Davidoff MD

The frontal CXR from a middle-aged female shows findings consistent with sarcoidosis (probably stage II) characterized by hilar adenopathy and mild interstitial disease noted abutting the minor fissure. Heart block and tachyarrhythmias are one of the more common manifestations of heart disease in patients with sarcoidosis of the heart. This patient required a dual lead pacemaker.
Ashley Davidoff MD
Pericardial Involvement
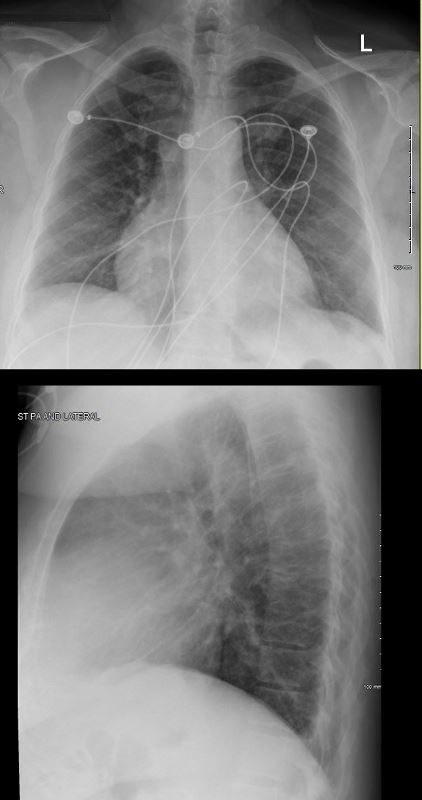
RECURRENT PERICARDIAL EFFUSION CXR,CT CORRELATION
54-year-old female with peripheral adenopathy who had an inguinal node biopsied 6 years prior showing sarcoidosis. Who chest examinations at the time showed little evidence of sarcoid except for a few small nodules. She has a history of hypertension COPD; sleep apnea, on CPAP; diabetes mellitus, on metformin; lumpectomy x2; GERD; and vertigo
4 years ago she presented with SOB and was noted to have a pericardial effusion by echo without tamponade
The pericardial effusion was drained with negative cytology and negative for TB . CT at the time following the drainage showed a thickened pericardium with pericardial drain in place
She represented 1 year later with symptoms of worsening intermittent chest pain, and shortness of breath exacerbated with exertion. An echocardiogram, showed recurrent increasing pericardial effusion confirmed by CT. She was placed on steroids but did not tolerate the steroids.
Later in that year she underwent surgery for pericardial window and VATS biopsy of her left lower lobe. Her pericardium was noted to be thickened
Pathology revealed Non-necrotizing granulomatous inflammation involving pulmonary interstitium and occasional airways; AFB, GMS, and PAS stains were negative for micro-organisms.
The left pericardial biopsy showed diffuse and extensive non-necrotizing granulomatous inflammation and AFB, GMS, and PAS stains were negative for micro-organisms.
CT scan at the end of that year (131555) showed thickened pericardium.
An MRI at the time showed diffuse thickening of the pericardium with enhancement as well as nodular mid myocardial changes at the hinge points and the inferolateral aspects. Subendocardial changes were also noted.
Repeat MRI the next year showed similar findings
Ashley Davidoff MD
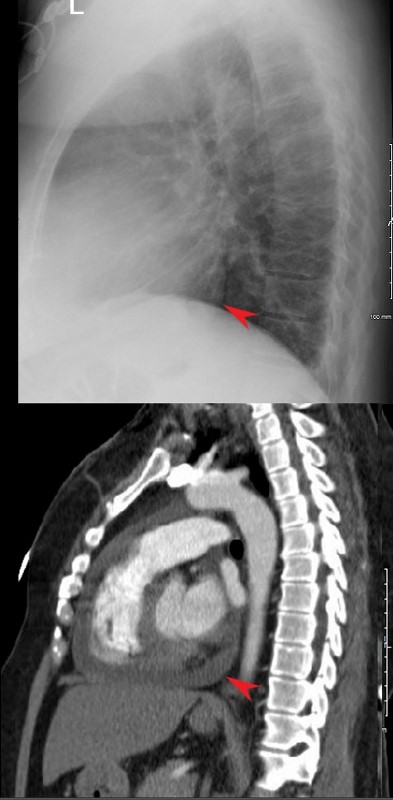
RECURRENT PERICARDIAL EFFUSION CXR,CT CORRELATION
The lateral examination soft pericardial effusion layering dependently and posteriorly.
Ashley Davidoff MD
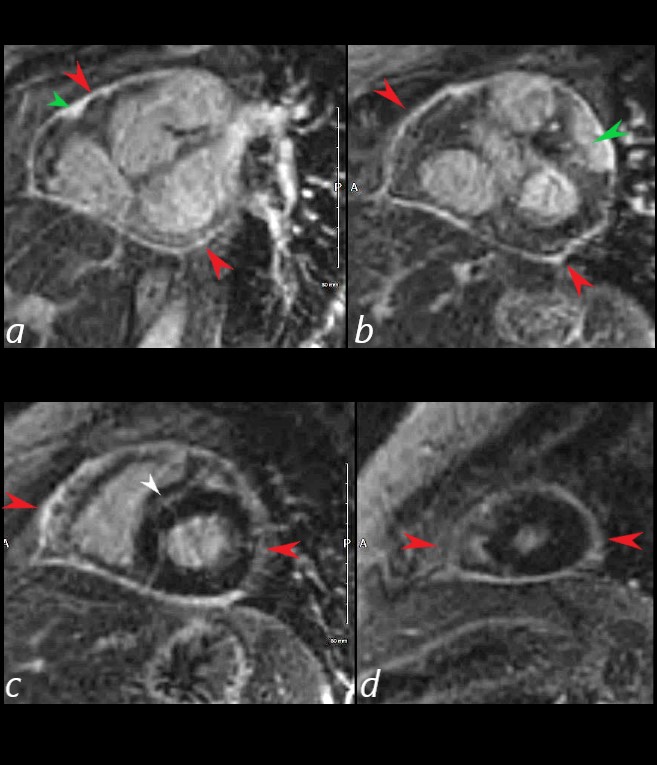
The short axis LGE sequence starting at the base of the heart (a, b) progressing through the body © and apex (d) shows focal LGE in the RVOT(a, green arrowhead) and in the LV (b green arrowhead) extensive pericardial enhancement (a,b,c,d ? red arrowheads, and in the septum (white arrowhead c).
54-year-old female with peripheral adenopathy who had an inguinal node biopsied 6 years prior showing sarcoidosis. Who chest examinations at the time showed little evidence of sarcoid except for a few small nodules. She has a history of hypertension COPD; sleep apnea, on CPAP; diabetes mellitus, on metformin; lumpectomy x2; GERD; and vertigo
4 years ago she presented with SOB and was noted to have a pericardial effusion by echo without tamponade
The pericardial effusion was drained with negative cytology and negative for TB. CT at the time following the drainage showed a thickened pericardium with pericardial drain in place
She represented 1 year later with symptoms of worsening intermittent chest pain, and shortness of breath exacerbated with exertion. An echocardiogram, showed recurrent increasing pericardial effusion confirmed by CT. She was placed on steroids but did not tolerate the steroids.
Later in that year she underwent surgery for pericardial window and VATS biopsy of her left lower lobe. Her pericardium was noted to be thickened
Pathology revealed Non-necrotizing granulomatous inflammation involving pulmonary interstitium and occasional airways; AFB, GMS, and PAS stains were negative for micro-organisms.
The left pericardial biopsy showed diffuse and extensive non-necrotizing granulomatous inflammation and AFB, GMS, and PAS stains were negative for micro-organisms.
CT scan at the end of that year (131555) showed thickened pericardium.
An MRI at the time showed diffuse thickening of the pericardium with enhancement as well as nodular mid myocardial changes at the hinge points and the inferolateral aspects. Subendocardial changes were also noted.
Repeat MRI the next year showed similar findings
Ashley Davidoff MD
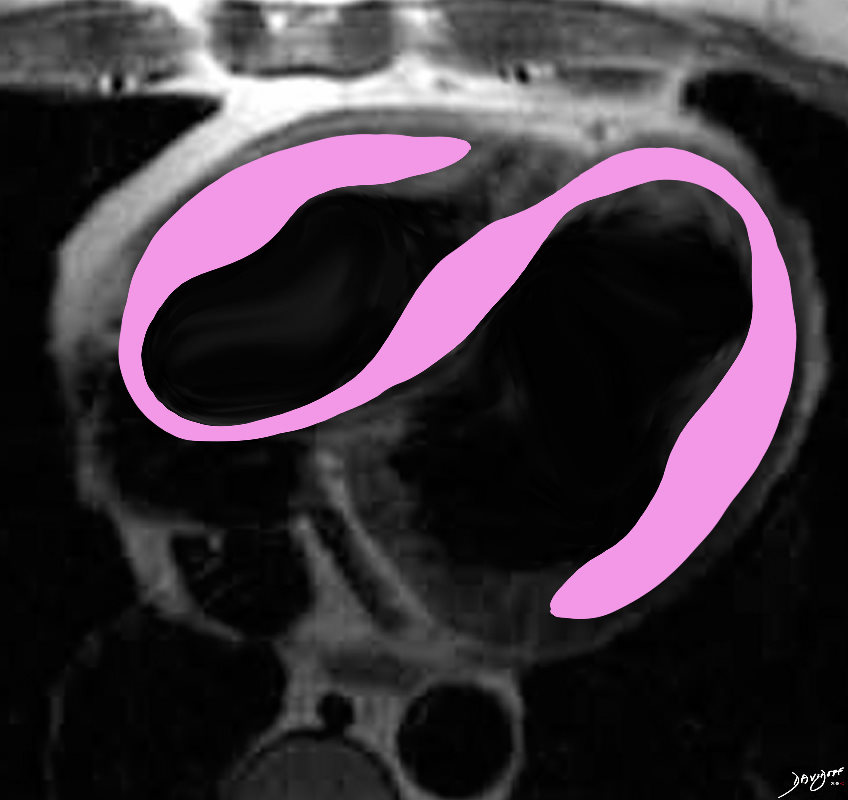
Sarcoidosis involves both ventricles and may cause bi-ventricular thickening
Ashley Davidoff MD
Cardiac Enlargement
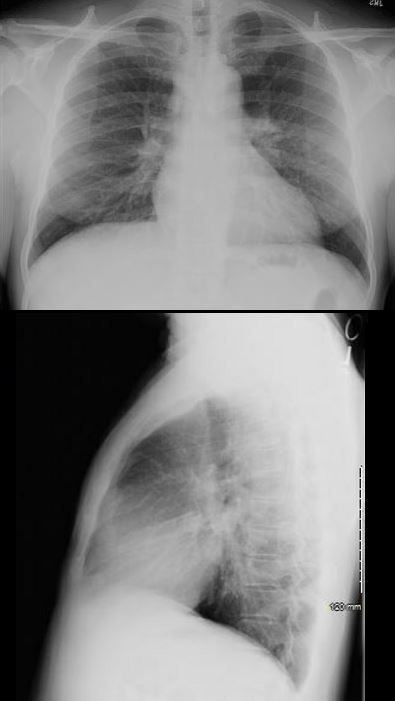
SARCOID CARDIOMYOPATHY with LIVER and LYMPH NODE INVOLVEMENT
57-year-old male with pathology findings consistent with granulomatous hepatitis and non-necrotizing granulomas in inguinal lymphadenitis both consistent with sarcoidosis but without pulmonary findings. Lymphadenopathy is present in the axillae and groins without involvement of the mediastinum
Associated cardiovascular findings include findings consistent with hypertrophic cardiomyopathy with:
o Diabetes and Hypertension
o CXR showing LVE
o Abnormal stress test with concerning regions in RCA territory inferiorly
o Moderate LVH on echo with normal EF (68%) normal LA, RA PAP and RV function. LV mass was 127g/sq. m
o Focal nodular LGE in the anterior apical region in mid myocardial/subendocardial region and in the inferior mid myocardial wall medially
o Subsequently developed episodes of paroxysmal ventricular tachycardia with EP ablation and placement of a defibrillator (ICD)
o LH and RH catheterization performed 6 years after initial studies showed elevation of PC WP of 25 mmHg, mean RA pressure of 16 mmHg, mean PAP of 34 mmHg no CAD
o Subsequent CT showed mildly enlarged LA and RA with mild TR
o 6 years after initial presentation he had symptoms of biventricular failure with increasing dyspnea and pedal edema culminating in an acute episode of monomorphic VT episode, ectopic atrial flutter/fibrillation, and left bundle branch aberrancy requiring amiodarone and cardioversion. Underwent upgrade to biventricular upgrade ICD
Ashley Davidoff MD
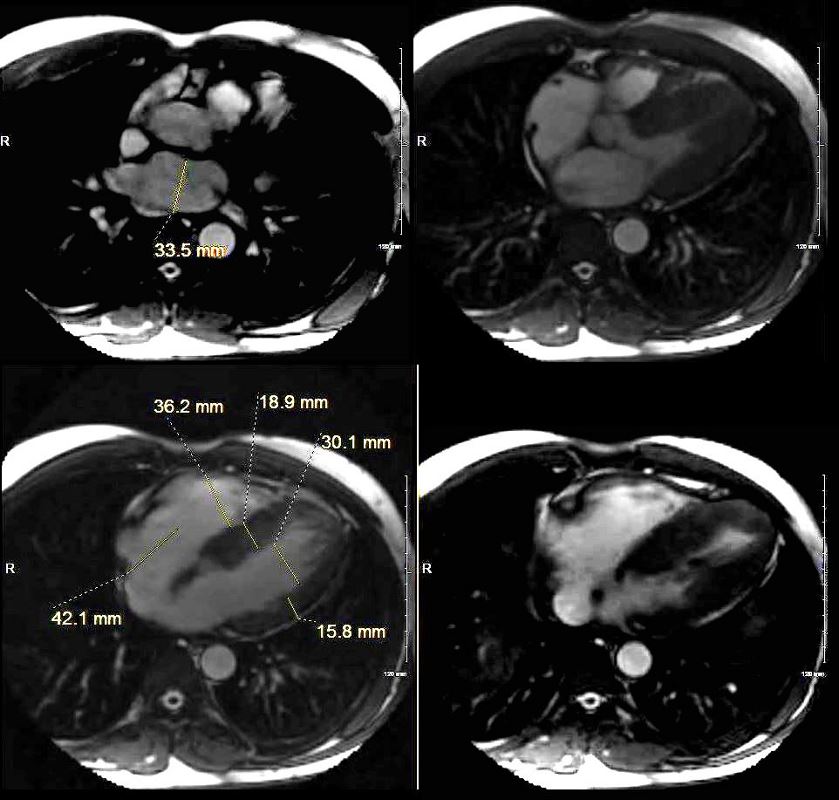
Ashley Davidoff MD
BiVentricular Thickening/Infiltration
LV MAss of 120g/sq m with extensive Myocardial Involvement
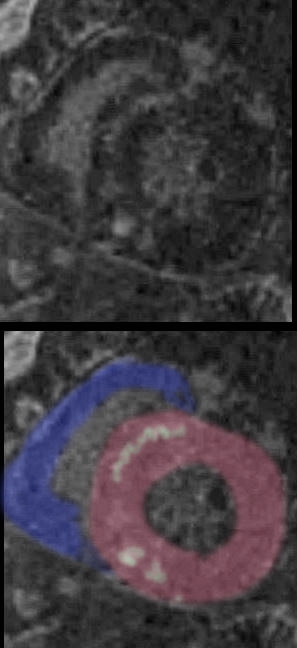
69 year old male presented with history of cardiomyopathy and atrial fibrillation
The LGE on the short axis shows biventricular thickening of the RV and LV
There are other findings on the MRI that are highly suggestive of sarcoidosis. There are multicentric foci of LGE in linear and nodular form in the mid myocardial and subepicardial layers and likely in the pericardium and myocardium of the right ventricle.
There was associated global hypokinesis of the LV with an EF of 40%, and increase in the LV mass of 120gms/ sq m
Ashley Davidoff MD

69 year old male presented with history of cardiomyopathy and atrial fibrillation
In this series the long axis 4 chambered view reveals intense continuous mid myocardial LGE
Ashley Davidoff MD
Cardiac sarcoidosis is an inflammatory disorder of the heart characterized by non-caseating granulomas in the myocardium The heart is involved in about 25% of patients with systemic sarcoidosis
Etiology
unknown
Structurally
The disease is patchy and multifocal. Te=he endocardium is spared.
Clinically
Most patient are asymptomatic but may present with conduction abnormalities and less frequently CHF
Diagnosis
Clinically if a patient has systemic sarcoidosis and cardiac symptoms are present there is a 50 % likelihood that card
Imaging
MRI and FDG -PET
MRI Typical LGE patterns in patients with cardiac sarcoidosis include patchy multifocal subepicardial and midwall LGE along the basal septum, sometimes with extension into the right ventricular insertion points as well as the infero-lateral wall.
MRI shows multifocal regions of LGE in the mid myocardium involving the inferobasal portion and inferolateral portion of the of the LV
Nonetheless, no specific pattern of LGE on CMR is diagnostic for cardiac sarcoidosis.
The presence of LGE in a non-infarct pattern may also be associated with fibrosis from prior myocarditis or idiopathic cardiomyopathy; rarely, cardiac sarcoidosis may mimic an infarct pattern.
CMR and FDG-PET evaluate different pathological processes, LGE evaluates fibrosis and FDG PEt evaluates acute inflammation by activated macrophages. Neither technique is specific cardiac sarcoidosis. These tests therefore provide complementary information
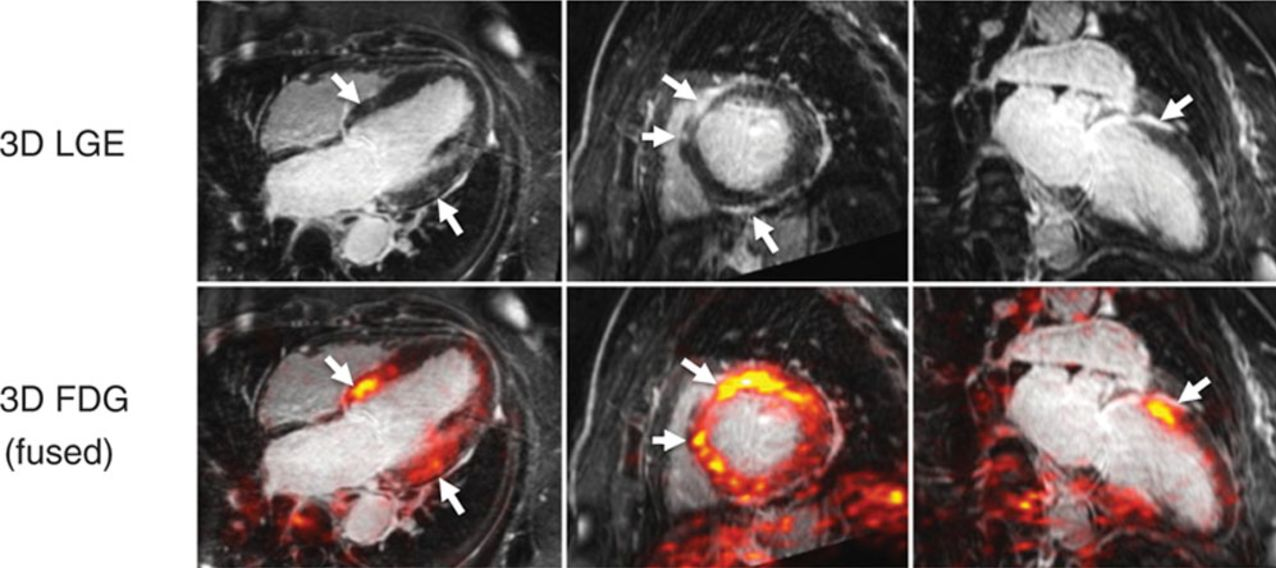
Courtesy https://www.acc.org/latest-in-cardiology/articles/2017/04/10/08/43/cardiac-mri-vs-pet
Both techniques provided high sensitivity of >75% for detection of cardiac sarcoidosis
Biopsy 30%
Sarcoidosis and Pericarditis

48 year old woman with path proven sarcoidosis of the lung presents with a moderate sized pericardial effusion. There is enhancement of the pericardium anterolaterally characterized by enhancing pericardium. Echo showed no tamponade. She was treated with anti-inflammatory medications and the pericardial effusion resolved . tentative diagnosis of pericarditis secondary to sarcoidosis
Prognosis
The extent of myocardial LGE is an important prognostic marker. Patients with cardiac sarcoidosis involving ?20% of left ventricular mass have higher risk of cardiac death, hospitalization for heart failure and life-threatening arrhythmias. Additionally they were less likely to demonstrate functional recovery of the left ventricle following steroid therapy.
References and Links
Ipek, E et al Sarcoidosis and the heart: A review of the literature Intractable Rare Dis Res. 2015 Nov; 4(4): 170?180.
Paco E. Bravo, MD; Viviany R Taqueti, MD, MPH, FACC Cardiac MRI vs. PET for the Evaluation of Cardiac Sarcoidosis: Consider MRI First
Shimada,T et al Structure and distribution of lymphatic capillaries and fenestrated blood capillaries in the conduction system of the rabbit heart Heart and Vessels volume 4, pages123?127(1988)
Cardiac Sarcoidosis Society of Thoracic Radiology