In chemistry and biology, units interact (<~>)with each other in a dynamic way rather than having a strictly additive (or subtractive) relationship. This infers a more give and take interaction. The interaction also has context, including a given environment, soace and time.
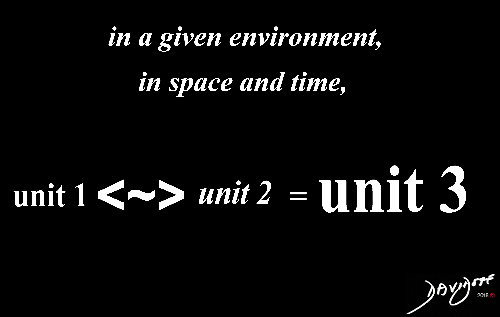
In Chemistry

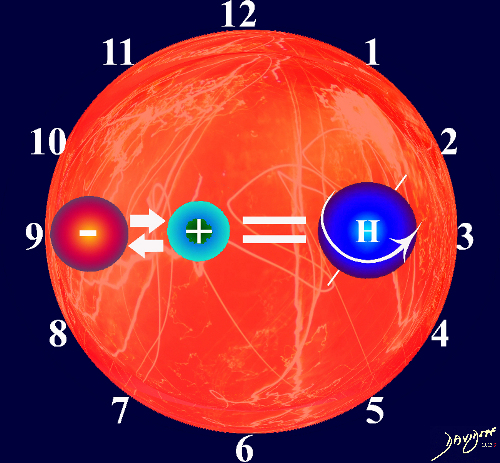

The 1+ 1 = 1 equation is still applicable, since units are additive in the formation of a bigger whole , and the end point “whole” of the interaction is still greater than the parts.
The “+” in the original equation throughout the common vein will be replaced by a variety of different bonding mechanisms between two units. For example in human biology the vessels including the arteries, veins, and ducts will bond two or more organs.

