Structural Considerations in Pain
Clinical Strategies
The Common Vein Copyright 2008
James Armstrong PA
Jessica Humphries
Ashley Davidoff MD
Structural Basis of Pain
A pain impulse is initiated by sensory receptors called nociceptors which are located in almost all the tissues. A noxious stimulus say from a hand touching a hot stove is then transmitted by sensory nerves to the spinal cord where a direct spinal reflex causes immediate withdrawal from the source. Additionally the stimulus is modified in the spinal cord by a variety of influences from other sources and is then transmitted via the midbrain and reticular activating system to the cortex. Finally, the stimulus reaches the brain’s somatosensory area where it is perceived and localized with additonal extension to other areas of the cortex for the provision of a variety of protective reactions to the stimulus.
We will now expand the detail of the structural pathway described above.
The Sensory Receptors
Nociceptors
Nociceptors are sensory neurons that are sensitive to painful or noxious stimulation. A nociceptor consists of free nerve endings which detect the stimulus, a long peripheral afferent fiber (peripheral process or primary afferent axon) that transmits the impulse to a ganglion cell, and a short fiber (central process) that takes the signal from the ganglion cell to the spinal cord.
 |
| Nociceptors and their Pathway to the Spinal Cord
The sensory receptors of the nociceptors are found in the tissues peripherally (top left) and are connected by a long fiber that transmits the impulse to the ganglion cell that lies in the dorsal ganglion in the neural canal alongside the spinal cord.
83066b09.8sb nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008 |
These specialized receptors vary in structure and number throughout the tissue and viscera of the body. There are external nociceptors that are situated in the skin and cornea with higher concentrations in the coverings of the body including the skin, pleura, pericardium, peritoneum and periosteum. Internal nociceptors are found in muscles, joints, around blood vessels, and within the mucosa of some organs including the urinary bladder, genitourinary tract, and the gastrointestinal tract. There are nociceptors in varying concentrations in almost every organ in the body, but interestingly there are none in the brain substance itself .
There are two types of fibers that conduct painful stimuli; A delta fibers and C fibers. The speed of conduction relates to the diameter of the nerve and the presence of a myelinated sheath. A delta fibers are thicker and have a thin myelinated sheath while the C fibers are unmyelinated. Sharp or pricking pain is transmitted by the faster, medium sized myelinated A delta fibers and which is responsible for the “immediate” pain experience, while the more prolonged aching aspect of pain is transmitted by the smaller non myelinated C fibers. Another type of fiber used in the modification of pain but not sensitive to pain itself is called the A beta fibers and they are heavily myelinated and thicker than A delta and C fibers. Hence, A beta fibers transmit their stimulus even faster.
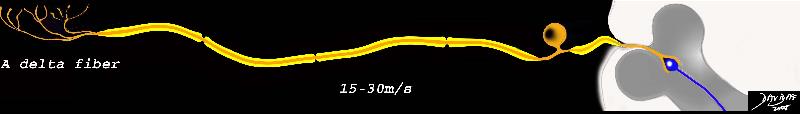
A delta Fibers
A delta fibers are sensory fibers that are structurally characterized by their intermediate size, thin myelin sheath, a single long peripheral fiber, a cell body located near the spinal cord in the dorsal root ganglion, and a short central fiber connecting the cell body to the dorsal root of the spinal cord.
Physiologically the receptors are sensitive to pressure, (mechanoreceptors), have a high threshold to pain, – specifically sharp pain (high threshold nociceptors) and extreme hot and cold temperatures (>45°C or <5°C).
In the context of pain A delta fibers are responsible for the immediate and first response to acute pain. They travel either to the spinal cord and cause a reflex muscle action to cause withdrawal from the immediate danger (hot plate example) and/or to the brain for the cognitive perception. Their lightly myelinated medium sized fibers allow conduction speeds of 5-30m/s. (about 40miles per hour)
Clinically the pain perceived from A delta fiber transmission can be variably localized depending on the concentration of receptors. Where nociceptors are high in concentration, localization can literally be pinpoint in accuracy. In areas of low concentration, the sensation may be felt over an area of 10-15cms. Either way, the localization of the pain is far better performed by the A delta fibers than that provided by the C fibers.
 |
|
A Delta Fiber
The A delta fiber consist of free nerve endings, are of intermediate size, are minimally myelinated (yellow sheath) and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
883066c02bd.8s This diagram shows A delta fiber whic is minimally myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 15-30m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008 |
C Fibers
C fibers are polymodal sensory fibers that are structurally characterized by their small size, absence of myelin sheath, a single long dendrite, a cell body located near the spinal cord in the dorsal root ganglion, and a short axonal connection to the spinal cord.
From a functional standpoint, their receptors are sensitive to a variety of stimuli, including mechanical, chemical, and thermal stimuli and in the context of pain are the secondary responders to pain. Their unique non myelinated fibers are slower conductors of the impulse which travels at about about 2m/second (approximately 3mph).
Clinically the pain perceived is throbbing or aching that immediately follows the hyperacute sharp pain, and in general is diffuse and sometimes even referred. However since the C fiber activates the reticular activating system on its route to the brain, it may be associated with other visceral responses including increased heart and respiratory rates, nausea, vomiting, loss of pallor and even fainting.
C Fiber |
| The C fiber are small in size, are non myelinated, and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
83066c02c.8s This diagram shows the two types of receptors and fibers that transmit the pain impulse. The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 2m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008 |
Detection of the Stimulus – Free Nerve Endings
The actual receptors of painful stimuli are branched free nerve endings on the most distal portion of the peripheral process of both the A delta and C fibers. There are a few varieties of receptors which are specific to the type of painful sensation they can sense. Thus there are mechanical, thermal, chemical, and polymodal nociceptors.
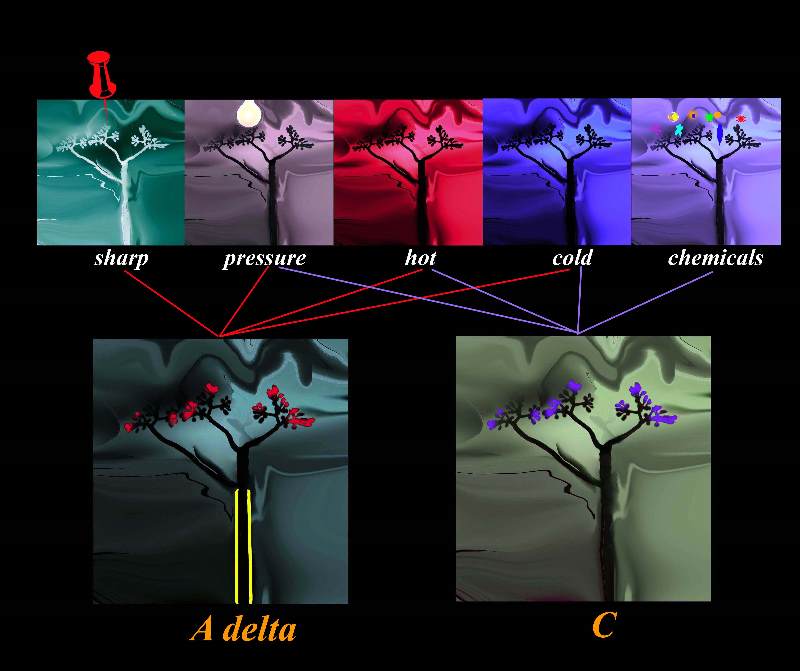 Types of Receptors Subtending the A delta Fibers and C Fibers Types of Receptors Subtending the A delta Fibers and C Fibers |
| The diagram shows sensory stimuli including sharp pressure, extreme heat and cold as well as chemical, stimulating the free nerve endings of the nociceptors that are linked to the myelinated A delta fiber , and non myelinated C fiber. The myelinated fiber will conduct the impulse between 3 and 15 times faster than the non myelinated fiber.
87559pc08b03.8s
pain free nerve ending nociceptor A delta fibres C fibres heat cold pressure mechanical prick afferent somatosensory nerves somatic anatomy normal Davidoff art Copyright 2008 |
All classes of nociceptors are present in the skin and tissues and work together in forming the pain response. For example, one may initially experience a feeling of “sharp” pain when hitting one’s thumb with a hammer, proceeded by a prolonged “aching.” The initial pain occurs when A delta fibers transmit information from mechanical and thermal nociceptors to the brain. C fibers transmitting polymodal nociceptors are responsible for the latent but more prolonged aching experience.
The Dorsal Root Ganglia
The dorsal root ganglion (or spinal ganglion) is a localized conglomerate of the cell bodies of sensory (afferent nerves) that are situated on the dorsal root of the peripheral nerves just before they enter the spinal cord. They can be found within the neural foramen of the bony vertebral column. The dorsal ganglion for the facial structures is called trigeminal ganglion and is located in the skull, while the dorsal ganglia for the rest of the body are located along the spine.
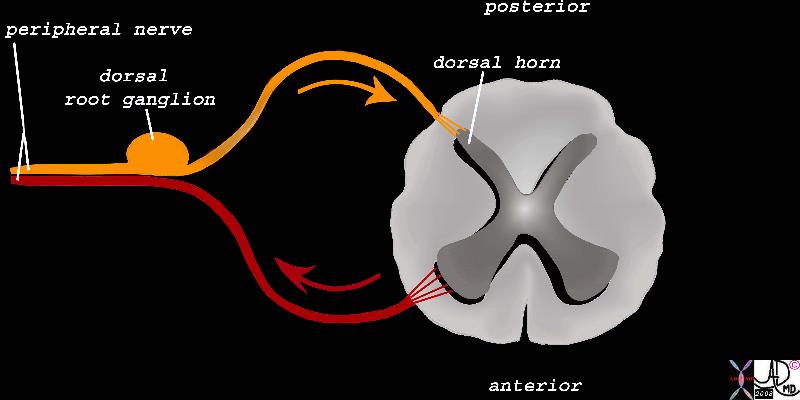 The Dorsal Root Ganglion of the Afferent Neurons The Dorsal Root Ganglion of the Afferent Neurons |
| The dorsal root ganglion is a focal accumulation of the first order nerve cells of the sensory component of the peripheral nerve. (orange) It is situated in the neural foramen of the vertebral body. The central process emanates from the ganglion cell and ends in the dorsal horn.
83067b03b04.8s peripheral nerve sensory nerve motor nerve dorsal root ganglion dorsal horn pain pathway spinal cord Davidoff Art Copyright 2008 |
1st 2nd and 3rd Orders of the Neurons
The propagation of a pain stimulus requires three orders of neurons. The first order is as described above and is the neuron that brings the stimulus from the periphery to the spinal cord. Second order sensory fibers cross to the contralateral side of the spinal cord and connect to the thalamus. Third order sensory neurons then connect the thalamus with the sensory cortex
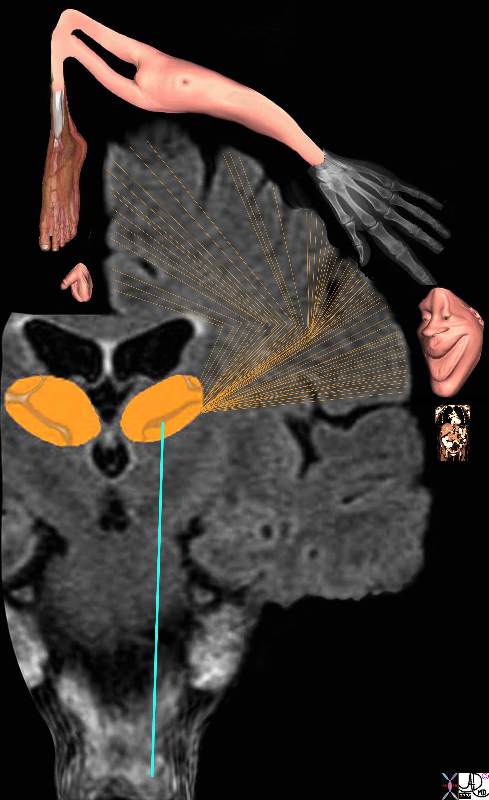
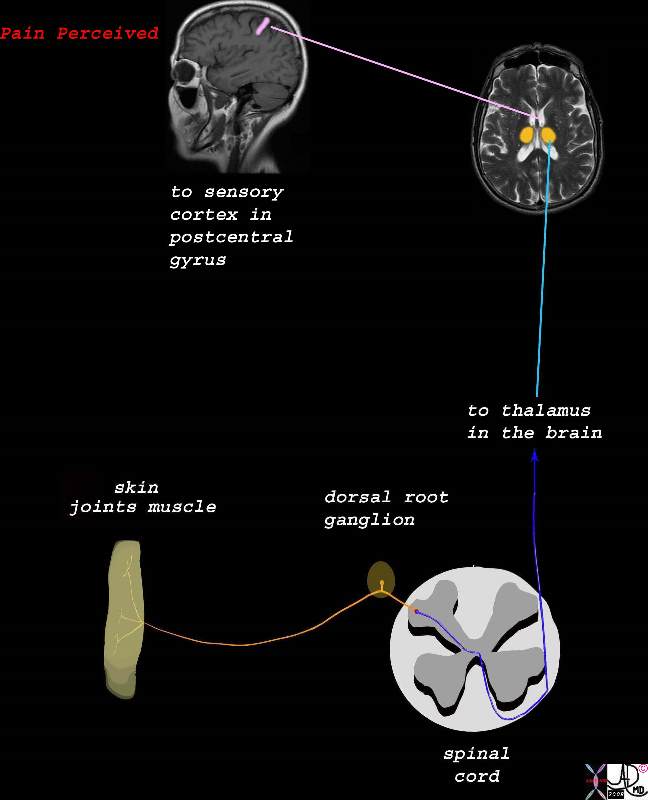 From the Spinal Cord to the Brain and Perception of the Pain From the Spinal Cord to the Brain and Perception of the Pain
The Three Orders of Neurons |
| The stimulus is converted into an electrical impulse which is taken by a first order sensory nerve (orange) to the spinal cord (dorsal root ) which in turn transports the impulse via second order neuron (dark blue and light blue) to the thalamus,. The third order neuron (pink) transports the impulse to the somatosensory cortex.
77533b06d06.8s pain skin joint muscle nociceptors dorsal root ganglion spinal cord brain thalamus sensory cortex somatosensory cortex postcentral gyrus pain perception localization perceive Davidoff art Copyright 2008 |
Role of the Spinal Cord
When the stimulus reaches the spinal cord it travels up and down for a couple of segments in the white matter of the dorsolateral tract of Lissauer before it enters the gray matter of the dorsal horn. There it is processed and “read ” and a variety of wave fronts are generated depending on peripheral, local, segmental and cortical influences. This process is called modulation occurs in the substantia gelatinosa, marginal nucleus, or nucleus proprius. The impulse may for example be transmitted via a spinal reflex to cause an immediate protective withdrawal response. The signal can also induce a local autonomic response. The impulse then crosses to the other side of the spinal cord via a second order neuron and travel in the white matter tract called the spinothalamic tract via the medulla, pons, and midbrain to the thalamus.
The Gate Control Theory
The gate control theory (Wall and Melzack) is a theoretical mechanism that proposes that there is modification of the pain stimulus in the spinal cord by the interaction of relative stimuli from A delta, C, and A beta fibers. Once the nociceptors have been stimulated, an electrical impulse is generated and transmitted to the ipsilateral side of the spinal cord via nerve fibers from the dorsal ganglion to the dorsal horn. It may travel and connect with other neurons one or two levels up or down thus diffusing the accuracy of localization. The electrical impulse is read in the context of other sensory input including non-nociceptive information from the other sensory nerves such as the A beta fibers. Therefore, if only the A delta pain fibres are stimulated, the gate is opened and pain is perceived. If there is additional input from non-nociceptive fibers, then the gate is closed to a variable degree and pain can be reduced. For example if a person suffers a bump on the head, the natural response is to vigorously rub the injured area. This induces and excites pressure receptors thus transmitting the impulse rapidly over heavily myelinated and large A beta fibers. This causes a relative closing of the gate in the spinal cord, dampening of normal pain impulse conduction, and thus a modification of a painful sensation to one of pressure.
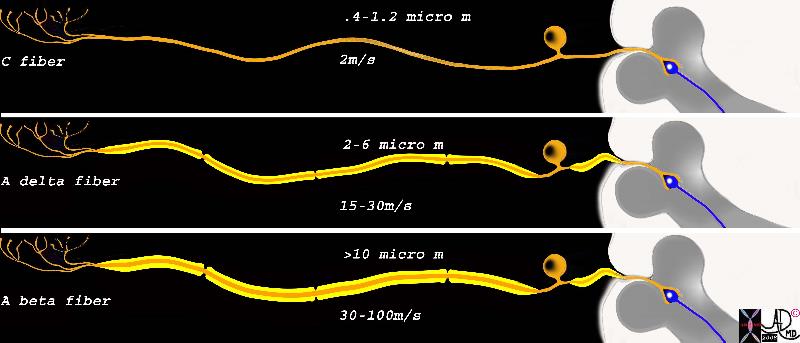 Fibers involved in Gate Control Mechanism Fibers involved in Gate Control Mechanism |
| This diagram shows the three types of receptors and fibers that transmit impulses related directly and indirectly to pain . The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, measuring between .4 to 1.2 micrometers, and conducts the impulse at about 2m/s. The second neuron is the A delta fiber and it responds to the pricking or sharp sensation that is first felt and reacted to. It is weakly myelinated and is about 2-6 micro meters thick, and conducts the stimulus with a velocity of between 15-30 meters per second. The last fiber is the A beta fiber and it is responsible for the pressure component which indirectly affects response to pain by affecting the gate mechanism of pain. It is greater than 10 microns thick due to heavier myelination and conducts impulses at 30-100meters per second
fiber neuron long peripheral process short central process ganglion cell ganglion body nerve sensory nerve dorsal ganglion dorsal column synapse Davidoff art Courtesy Ashley Davidoff MD Copyright 2008 83066c05b.8s |
2nd order neurons and the Spinothalamic tract
As described above, the second order neurons conduct the stimulus from the ipsilateral side of the stimulus to the contralateral white matter and the major pathway is then via the spinothalamic tract to the thalamus.
The spinothalamic tract is the major sensory ascending pathway of 2nd order neurons and serves as the major pathway for pain, temperature, itch and crude touch. Within its construct, the spinothalamic tract has three merging bands of specialized fibers that conduct characteristic pain impulses. The anterior spinothalamic tract carries pain signals initiated by touch while the lateral spinothalamic tract carries slow and fast fibers for pain and temperature sensations. The anterolateral spinothalamic pathway, located in the anterolateral white column pathway in the anterior half of the lateral funiculus conducts a variety of somatic pain signals.
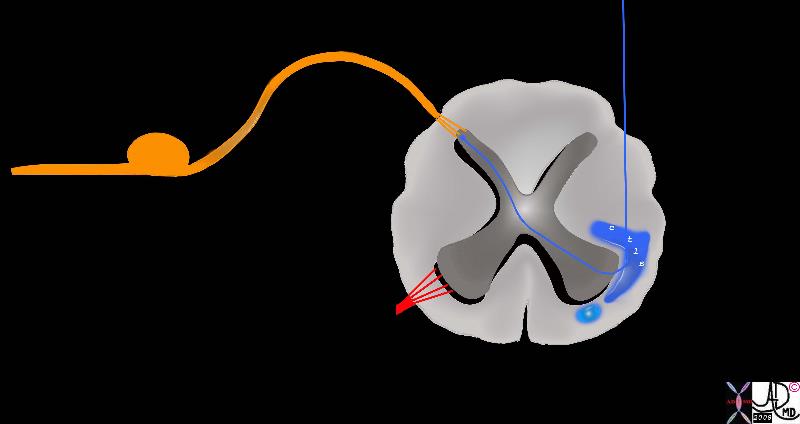 Second Order Neurons Second Order Neurons
Cross Over and entry into the Spinothalamic Tract |
| The pain fibres cross over the spinal cord via the second order neuron (blue) to the spinothalamic tract. The lateral spinothalamic tract and the anterior spinothalamic tract There are two parts to the anterolateral spinothalamic tract. The lateral spinothalamic tract (darker blue) carries the fibers for pain and temperature sensations and the anterior spinothalamic tract (light blue) carries sensation of simple touch.
orange = sensory nerve carrying stimuli from periphery
blue = anterolateral spinothalamic tract
dark blue = lateral spinothalamic tract
light blue = anterior spinothalamic tract
Davidoff art Courtesy Ashley Davidoff MD copyright 2008 83067b05b05.8s |
En route to the thalamus, the second order neurons have to pass through the medulla, pons and midbrain. One of the systems that is activated by the C fibers in this path is the reticular activating system.
C Fibers and the Reticular Activating System
The reticular activating system (aka RAS, ascending reticular activating system) is a part of the brain considered to be the center of arousal and motivation. Structurally it lies between the medulla oblongata and midbrain and is connected to the thalamus. In turn, all parts of the brain can be stimulated by the RAS including the cerebral cortex basal regions of the brain and the medulla. Functionally, the RAS indirectly relates to our state of consciousness, and is involved with the control of the circadian rhythm, respiration, cardiac rhythms, and sexual function. In the instance of pain, the RAS is activated by the C fibers and hence enables a painful stimulus to arouse us from sleep, create a sense of urgency, and can cause changes in heart rate or respiration rate.
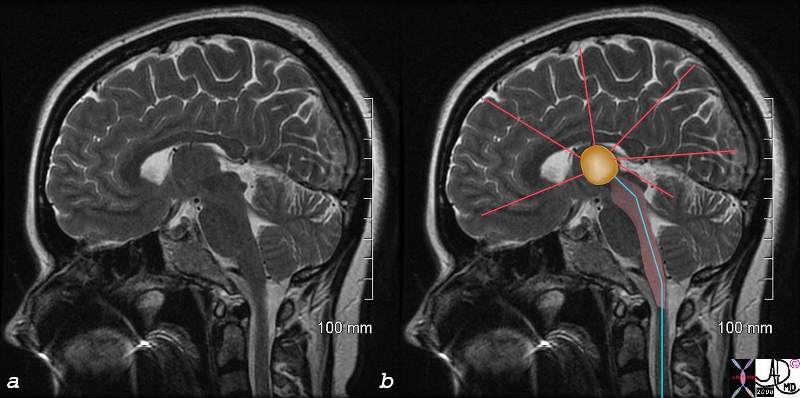 C Fibers and the Reticular Acivating System C Fibers and the Reticular Acivating System |
| The image represents a coronal cut of the brain attained using T2 weighted MRI technique. It reveals a second order neuron (blue) traversing the medulla, pons, and midbrain, and in its path the C fiber component, is able to activate the RAS (pink). The stimuli reach the thalamus (orange) which not only activates the sensory cortex but other parts of the cortex as well as shown by the red lines.
C fibers pain brain reticular activating system RAS thalamus cortex medulla oblongata midbrain thalamus MRI T2 weighted Courtesy Ashley DAvidoff MD copyright 2008 77059c02.8s |
Role of the Thalamus
The thalamus is the gateway to the cerebral cortex. It is a paired organ and represents a major portion of the diencephalon.
Structurally, the thalamus has specific nuclei with diffuse projections to and from multiple regions of cerebral cortex.
The thalamus functions as a translator for the cerebral cortex. It processes sensory and motor information and mediates the autonomic nervous system regulating sleep and arousal. The thalamus also contains reciprocal connections to the cortex that are involved in consciousness. It may also play a role in vestibular function.
The thalamus translates pain signals of the 2nd order neurons and gives rise to the third order neurons that extend to the cortex. Awareness and localization of the pain is then achieved at the level of the cortex. The thalamus however is not merely a relay station for nociception but also plays a role in processing the stimulus.
Axons terminating in the lateral thalamus mediate discriminative aspects of pain (somatosensory cortex) including the originating body part. The fibers ending in the medial thalamus mediate the motivational and affective aspects relating for example to the emotional and memory of pain. These third order neurons travel to the prefrontal cortex, insular and cingulate gyrus which contribute to the emotion and memorization of pain experiences.
Somatosensory Cortex in the Parietal Lobe
The somatosensory cortex is part of the somatosensory system and is characterized by its parietal location in the brain and by its ability to perceive and localize the pain.
Structurally, the cortex lies as the anterior most structure of the parietal lobe, positioned between between the motor cortex of the frontal lobe and the central sulcus anteriorly, the post central sulcus posteriorly, and the lateral sulcus inferiorly.
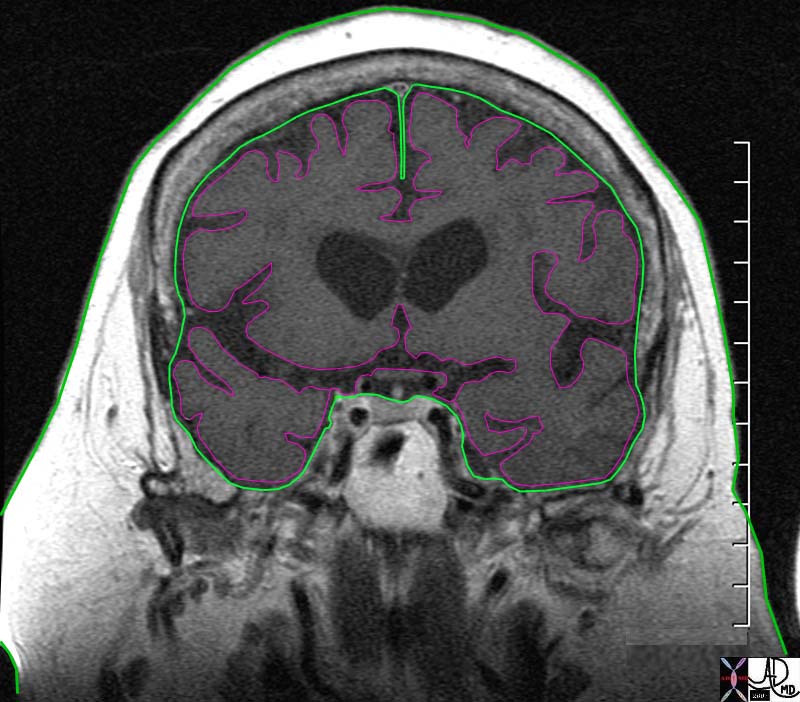
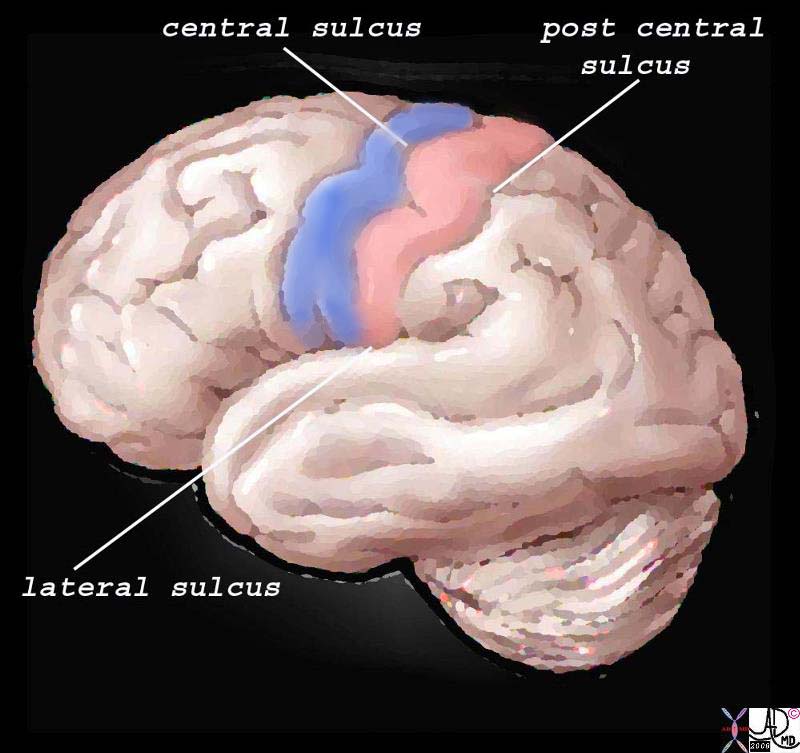 The Somatosensory Cortex – Post Central Gyrus The Somatosensory Cortex – Post Central Gyrus |
| The somatosensory cortex is overlaid in light rose pink in the diagram above and represents the most anterior structure of the parietal lobe. It lies posterior to the motor cortex (blue) which is part of the frontal lobe, behind the central sulcus and in front of the post central sulcus. It serves to perceive, localize and evaluate intensity of the pain, as well as initiate the response to the pain.
83029b01.b1.81s brain somatosensory cortex pareital lobe medial longitudinal fissure medially central sulcus anteriorly postcentral sulcus posteriorly lateral sulcus inferiorly location of primary somatosensory cortex main sensory receptive area touch. maps sensory space homunculus in this location pink = somatosensory cortex in post central gyrus blue = motor cortex The Common vein Davidoff art copyright 2008 |
The regions of the body have a specific location in the somatosensory cortex and, depending on the number of nociceptors, will have a correlative sized distribution. Thus for example, the lips, mouth, hands, feet, and genitalia will have a much larger representation in the somatosensory cortex than the limbs, trunk, and viscera. Subsequently, the various structures have a descending order of representation and consequently a descending order of sensitivity. The homunculus figure represents this concept and is diagrammed below.
If the somatosensory cortex is viewed in the coronal plane, the homunculus (literally “little man”) is draped over the sensory cortex with genitalia, and legs draped medially, thighs and trunk superiorly, and hands, head, mouth, lips, pharynx, tongue and viscera draped laterally. The size of organ representation is not only specific to pain fibers but related to the sensory and motor system as a whole.
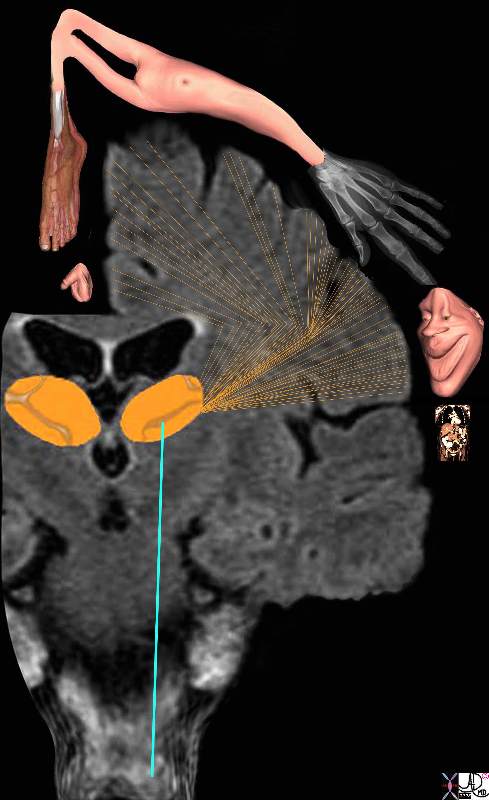  Somatosensory Cortex in the Parietal Lobe Somatosensory Cortex in the Parietal Lobe
Localization and the Homunculus Man |
| The diagram reflects the relative functional sensory space each body part occupies in the somatosensory cortex. Those structures with high density of sensory receptors are represented by a larger size, while those with a lesser concentration of sensory apparatus shown as being “smaller” in size. Hence the mouth lips, hands feet and genitalia have a relatively large representation. The homunculus man (literally the “little man”) is the distorted figure drawn to reflect the concept of size of organ paralleling the size of the sensory innervation.
somatosensory cortex (sensory homunculus) spinothalamic tract spinal cord thalamus sensory cortex homunculus man penis clitoris genitals genitalia foot body thigh abdomen chest and face mouth eyes lips viscera somatosensory Davidoff art Copyright 2008 38610b09.46k.8s |
The function of the somatosensory cortex is that of a higher processing center for touch, temperature, pain, and proprioception serving to amplify awareness of the sensations enabled by the thalamus. Sensation from the left side of the body are processed in the right somatosensory cortex and similarly those from the right side are processed on the left. The higher function of the somatosensory cortex allows us to localize the pain to a specific site, perceive the character and intensity of the stimulus, and sometimes helps identify the shape of the originating object.
The somatosensory cortex is not the final level of the somatosensory system since it also relays impulses to other cerebral areas of perception and reaction. Thus it sends signals via the white matter to other centers in the cortex to enable integration with visual and auditory input, and with other higher cortical functions such as emotion and memory for example. The full experience is then “seen” by the brain enabling the consequent reaction to be as discriminating and prudent as the nature and experience of the person allows. The difference between the reaction of an infant, child and an adult to the “shot at the doctors” speaks volumes about this latter function.
Anatomical Regions of Pain
Head and Neck
Pain syndromes of the head and neck vary according to the degree and area of injury but are generally poorly tolerated due to the anatomical confines of these regions. The head and neck anatomy is highly vascularized and well innervated with both somatic and cranial nerve fibers. Pain symptoms therefore are often accompanied by neurological findings. Causes of head and neck pain include vascular disorders, trauma, neoplasm, infection and treatment related syndromes after surgery or radiation therapy.
Headache is defined as pain in the head that is located above the eyes or the ears, behind the head (occipital), or in the back of the upper neck. There are many causes for headache but among the most common causes are migraine and tension headaches. The vast majority of headaches are benign and self-limiting and result spontaneous resolution. Treatment is symptomatic aided with the use of appropriate analgesics. The most feared headache is the life threatening event of a ruptured berry aneurysm. It is estimated that three out of four Americans had a headache at least once during the past year, and approximately forty-five million Americans suffer from chronic headaches, accounting for 80 million doctors’ office visits and more than 400 million dollars spent on over-the-counter pain relievers each year.
Neck pain is a common complaint seen in various medical settings including in primary care and the emergency department. It has many causes including dysfunction of the muscles and supporting structures such as bones and their joints (musculoskeletal dysfunction), infection, neurological disorders, pain from somewhere else in the body (referred pain, as in headaches or heart attack), and disorders of the various other structures in the neck including the esophagus and thyroid. Most neck pain is related to musculoskeletal dysfunction: in the US, almost 85% of all neck pain is thought to result from neck injuries (either acute or recurring) or from chronic stresses such as prolonged computer use. In the general population, the 1-year prevalence rate for neck and shoulder pain is 16-18% (Dreyer, 1998) and about two thirds of people will experience neck pain at some time in their lives.
  Distribution of Somatic Nociceptors (green) and Visceral Nociceptors (pink) in the Head Distribution of Somatic Nociceptors (green) and Visceral Nociceptors (pink) in the Head |
| 71422.800b01b brain meninges pia mater = pink dura mater = green subarachnnoid space bone somatosensory receptors rich in the dura inner outer pain periosteum MRI Courtesy Ashley DAvidoff Copyright 2008 |
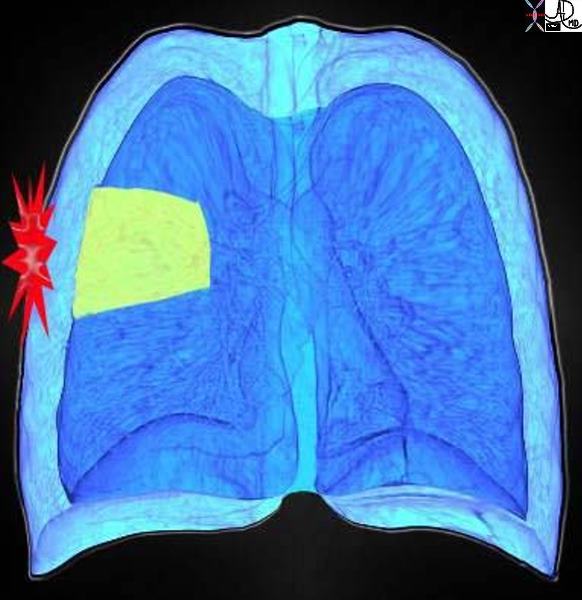
Chest
Due to the prevalence of cardiovascular disease and its common manifestation of thoracic symptoms, chest pain receives priority status when described by the presenting patient. Chest pain is defined as the painful sensation that is classically felt in the front part of one’s body, between the neck and the upper abdomen. It can have many causes, that range from life threatening conditions such as myocardial infarction and aortic dissection, to more benign conditions such as indigestion, heartburn, and gastoesophageal reflux, costochondritis, and muscular aches. We do however include in the module back pain that may reflect significant chest disease such as pulmonary embolism, pleurisy, and aortic dissection, while also sometimes reflecting abdominal disease such cholecystitis and kidney stones.
Chest pain sometimes does not result in sufficient discomfort for the patient to seek medical attention, as unfortunately serious disease is not always associated with severe pain.
Diagnosis of the underlying causes often is the result of a careful clinical history and physical examination.Triage to subsequent diagnostic investigation usually includes a chest X-ray, EKG and cardiac enzymes.
Medical therapy for chest pain causes includes analgesics, antacids, anticoagulation and intravenous thrombolytics. Minimally invasive techniques for atherosclerotic disease may involve endovascular therapies like angioplasty, and stenting. Surgical interventions consist of coronary artery bypass and repair of dissections.
Abdomen
Excluding direct trauma, abdominal pain is often visceral in nature and therefore difficult to delineate. Visceral pain receptors are stimulated by distension, contraction, inflammation and ischemia. An understanding of these characteristics and a sound knowledge of abdominal anatomy can be helpful in demystifying abdominal pain.
The abdomen is a complex structure consisting of multiple spaces and compartments filled with variety of heterogeneous organs and structures. The spaces and organs have been compartmentalized, divided and reclassified throughout the course of medical history according to the purposes of the specific group. For the clinician, dividing the abdomen into quadrants makes clinical sense. Right upper quadrant symptoms, for example bring certain differential considerations and these are completely different from left lower quadrant symptoms. For the clinician who thinks embryologically, foregut, midgut and hindgut division makes intuitive sense. For the surgeon who has to decide about an invasive approach, division of the abdomen relates to upper or lower, and right midline or left. The approach mostly depends on the clinical presentation and can be divided into either a focused or global approach.
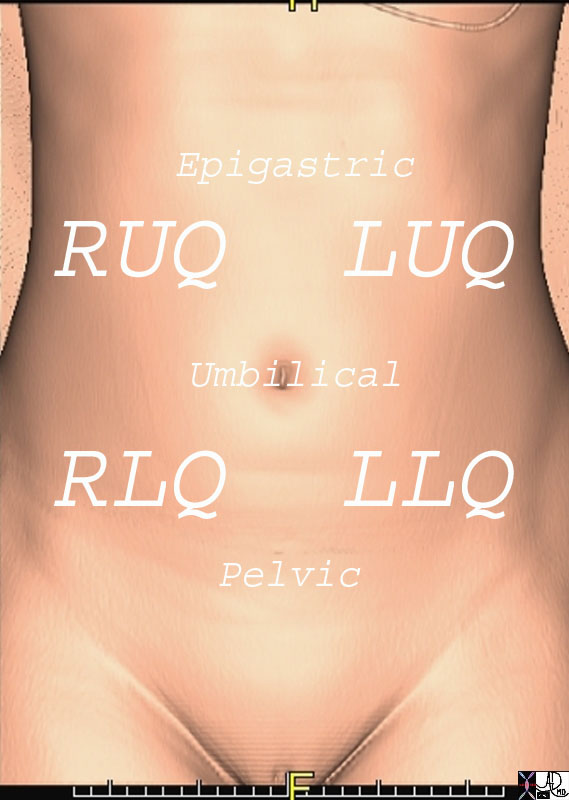 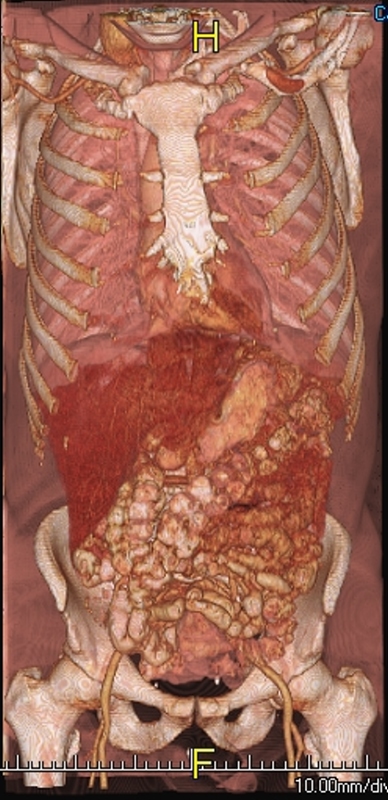 Quadrants of the Abdomen Quadrants of the Abdomen |
| 49703b19 71203.800 chest abdomen pelvis sternum bone ribs iliac crest femur femoral neck pubic symphisis ischium ischial tuberosity scapula clavicle lung heart pericardium liver stomach small bowel colon femoral artery normal anatomy CTscan 3D surface rendering Courtesy Ashley Davidoff MD |
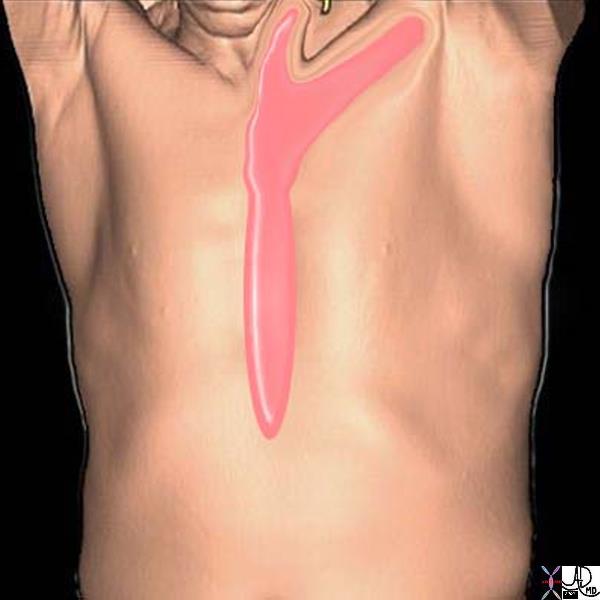
Pelvic
The pelvis is another highly vascularized area comprised mostly of the reproductive organs and a multitude of lumbar and sacral nerve plexi. Due to its anatomical proximity to the abdominal viscera, pelvic pain may often reflect an abdominal process.
 Acute Pelvic Pain Awakening the Patient Acute Pelvic Pain Awakening the Patient |
| 89081pd04b09.8s pain abdomen sleep woken awakened from sleep somatic peritoneal peritonitis Davidoff art copyright 2008 |
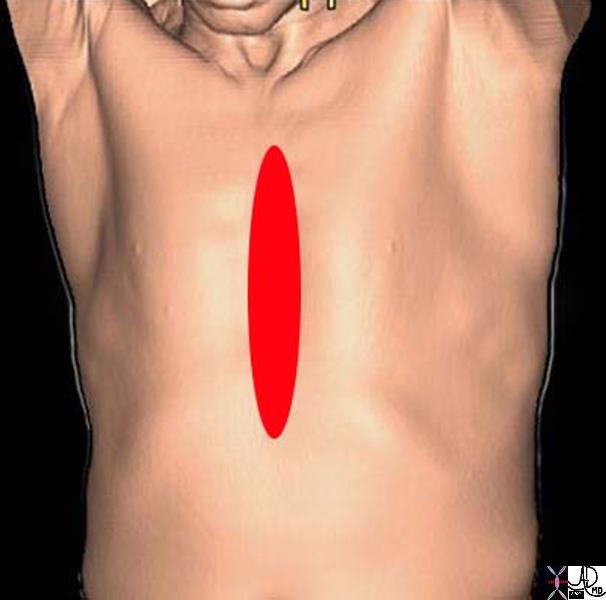
Back
Back pain is significant in its societal frequency and impact on the workforce. There is a ninety percent lifetime incidence of back pain in the adult population and it ranks second only to the common cold for worker absenteeism. Although classically back pain is a self-limited manifestation of muscle spasm in response to injury, disorders of the spinal structures and subsequent nerve roots can cause chronic, life altering pain and disability.
Back pain is a disturbing and uncomfortable sensation felt in the lower or upper back. Low back pain may be caused by structural or functional disorders of the lumbar spine, intervertebral discs, nerve roots, spinal cord, muscles or ligaments. The pain may also originate from the bony pelvis or pelvic organs. Sometimes disorders in the upper abdomen can present with back pain such as gallbladder disease, kidney and pancreatic disease. Lastly the skin of the back can also be the cause of back pain.
The clinical result ranges from fleeting pain to debilitating disease and sometimes to life threatening disorders.
The diagnosis requires a careful clinical history that focuses on precipitating factors, duration, onset, character, situation, severity, aggravating relieving, and associated disorders relating to the pain. Imaging may include plain X-ray films, CT scan, MRI or bone scan.
Treatment depends on the cause of the back pain and ranges from symptomatic relief with bed rest, analgesics and anti-inflammatory medication through physical therapy, to surgery when indicated.
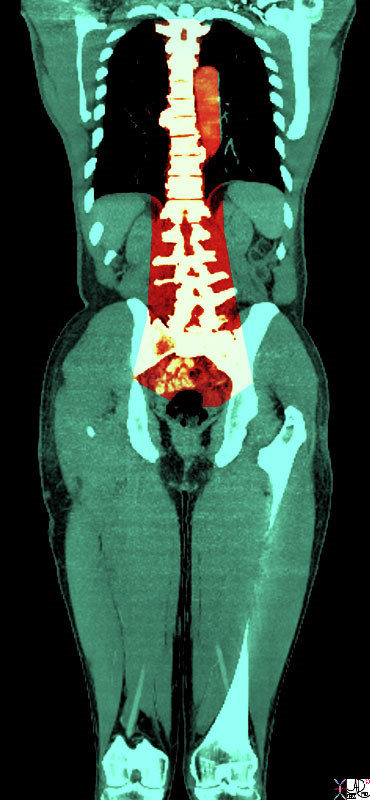
Anatomic Distributiuon of Back Pain
|
| Back pain has a wide distribution and includes structures of the thoracolumbar spine, chest and abdominal cavities.
48390.83 bone back pain spine lumbar spine thorax thoracic cage pancreatitis aortic syndrome CTscan Courtesy Ashley Davidoff MD |
Extremity
The most common cause of extremity pain is injury to soft tissue, bone or cartilage that is either acute or repetitive. Pain radiation is common in the extremities due to alignment of sensory and motor nerves within the fascia. For example, repetitive stress injury to the forearm causing tendonitis in tennis players is often manifested as elbow pain thus invoking the nickname “tennis elbow”. Degenerative joint disease in the elderly is a frequent source of chronic pain and disability often with surgical joint replacement as the only remedy.
References
Melzack, R., Wall, P.D. “Pain mechanisms: A new theory,” Science, 150:171-9, 1965.
Wall, P.D, Melzack, R. “On nature of cutaneous sensory mechanisms,” Brain, 85:331, 1962.
Purves D Fitzpatrick DWilliams MS, McNamara jO Augustine GJ, Katz lC, laMantia A S. Neuroscience second edition © 2001 by Sinauer Associates, Inc.
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Anatomic Distributiuon of Back Pain
Back pain has a wide distribution and includes structures of the thoracolumbar spine, chest and abdominal cavities.
48390.83 bone back pain spine lumbar spine thorax thoracic cage pancreatitis aortic syndrome CTscan Courtesy Ashley Davidoff MD
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Anatomic Distributiuon of Back Pain
Back pain has a wide distribution and includes structures of the thoracolumbar spine, chest and abdominal cavities.
48390.83 bone back pain spine lumbar spine thorax thoracic cage pancreatitis aortic syndrome CTscan Courtesy Ashley Davidoff MD
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Back pain has a wide distribution and includes structures of the thoracolumbar spine, chest and abdominal cavities.
48390.83 bone back pain spine lumbar spine thorax thoracic cage pancreatitis aortic syndrome CTscan Courtesy Ashley Davidoff MD
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Back pain has a wide distribution and includes structures of the thoracolumbar spine, chest and abdominal cavities.
48390.83 bone back pain spine lumbar spine thorax thoracic cage pancreatitis aortic syndrome CTscan Courtesy Ashley Davidoff MD
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
Anatomic Distributiuon of Back Pain
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
Anatomic Distributiuon of Back Pain
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Acute Pelvic Pain Awakening the Patient
89081pd04b09.8s pain abdomen sleep woken awakened from sleep somatic peritoneal peritonitis Davidoff art copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Acute Pelvic Pain Awakening the Patient
89081pd04b09.8s pain abdomen sleep woken awakened from sleep somatic peritoneal peritonitis Davidoff art copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] =>
[lastElementChild] =>
[childElementCount] => 0
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => 89081pd04b09.8s pain abdomen sleep woken awakened from sleep somatic peritoneal peritonitis Davidoff art copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => 89081pd04b09.8s pain abdomen sleep woken awakened from sleep somatic peritoneal peritonitis Davidoff art copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Acute Pelvic Pain Awakening the Patient
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Acute Pelvic Pain Awakening the Patient
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Quadrants of the Abdomen
49703b19 71203.800 chest abdomen pelvis sternum bone ribs iliac crest femur femoral neck pubic symphisis ischium ischial tuberosity scapula clavicle lung heart pericardium liver stomach small bowel colon femoral artery normal anatomy CTscan 3D surface rendering Courtesy Ashley Davidoff MD
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Quadrants of the Abdomen
49703b19 71203.800 chest abdomen pelvis sternum bone ribs iliac crest femur femoral neck pubic symphisis ischium ischial tuberosity scapula clavicle lung heart pericardium liver stomach small bowel colon femoral artery normal anatomy CTscan 3D surface rendering Courtesy Ashley Davidoff MD
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] =>
[lastElementChild] =>
[childElementCount] => 0
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => 49703b19 71203.800 chest abdomen pelvis sternum bone ribs iliac crest femur femoral neck pubic symphisis ischium ischial tuberosity scapula clavicle lung heart pericardium liver stomach small bowel colon femoral artery normal anatomy CTscan 3D surface rendering Courtesy Ashley Davidoff MD
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => 49703b19 71203.800 chest abdomen pelvis sternum bone ribs iliac crest femur femoral neck pubic symphisis ischium ischial tuberosity scapula clavicle lung heart pericardium liver stomach small bowel colon femoral artery normal anatomy CTscan 3D surface rendering Courtesy Ashley Davidoff MD
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Quadrants of the Abdomen
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Quadrants of the Abdomen
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Characteristics of Chest Pain
In the above diagram and through the text, we attempt to depict the nature of the pain diagrammatically. The more severe pains are shown in brighter red and the spiculated shapes reflect sharper pain. Thus the top left image is severe burning retrosternal pain characteristic of GERD with esophagitis. The top middle image is a pressure type pain that radiates o the neck, characteristic of angina and sometimes seen in esophageal spasm. The top right image is a more diffuse discomfort or pressure and is seen in angina and myocardial infarction. The bottom left image is sharp lancinating severe almost devastating pain characteristic of acute aortic dissection. The focal sharp pain in the middle image aggravated by deep inspiration is characteristic of pleuritc pain and pericarditis, while the pain along a dermatome on bottom right is seen in herpes zoster (shingles).
71197c05a 71197c07a 42540c02a05 71197c08a 71197b03p 71197c04a
Davidoff Art Courtesy Ashley Davidoff MD Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Characteristics of Chest Pain
In the above diagram and through the text, we attempt to depict the nature of the pain diagrammatically. The more severe pains are shown in brighter red and the spiculated shapes reflect sharper pain. Thus the top left image is severe burning retrosternal pain characteristic of GERD with esophagitis. The top middle image is a pressure type pain that radiates o the neck, characteristic of angina and sometimes seen in esophageal spasm. The top right image is a more diffuse discomfort or pressure and is seen in angina and myocardial infarction. The bottom left image is sharp lancinating severe almost devastating pain characteristic of acute aortic dissection. The focal sharp pain in the middle image aggravated by deep inspiration is characteristic of pleuritc pain and pericarditis, while the pain along a dermatome on bottom right is seen in herpes zoster (shingles).
71197c05a 71197c07a 42540c02a05 71197c08a 71197b03p 71197c04a
Davidoff Art Courtesy Ashley Davidoff MD Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => In the above diagram and through the text, we attempt to depict the nature of the pain diagrammatically. The more severe pains are shown in brighter red and the spiculated shapes reflect sharper pain. Thus the top left image is severe burning retrosternal pain characteristic of GERD with esophagitis. The top middle image is a pressure type pain that radiates o the neck, characteristic of angina and sometimes seen in esophageal spasm. The top right image is a more diffuse discomfort or pressure and is seen in angina and myocardial infarction. The bottom left image is sharp lancinating severe almost devastating pain characteristic of acute aortic dissection. The focal sharp pain in the middle image aggravated by deep inspiration is characteristic of pleuritc pain and pericarditis, while the pain along a dermatome on bottom right is seen in herpes zoster (shingles).
71197c05a 71197c07a 42540c02a05 71197c08a 71197b03p 71197c04a
Davidoff Art Courtesy Ashley Davidoff MD Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => In the above diagram and through the text, we attempt to depict the nature of the pain diagrammatically. The more severe pains are shown in brighter red and the spiculated shapes reflect sharper pain. Thus the top left image is severe burning retrosternal pain characteristic of GERD with esophagitis. The top middle image is a pressure type pain that radiates o the neck, characteristic of angina and sometimes seen in esophageal spasm. The top right image is a more diffuse discomfort or pressure and is seen in angina and myocardial infarction. The bottom left image is sharp lancinating severe almost devastating pain characteristic of acute aortic dissection. The focal sharp pain in the middle image aggravated by deep inspiration is characteristic of pleuritc pain and pericarditis, while the pain along a dermatome on bottom right is seen in herpes zoster (shingles).
71197c05a 71197c07a 42540c02a05 71197c08a 71197b03p 71197c04a
Davidoff Art Courtesy Ashley Davidoff MD Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Characteristics of Chest Pain
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Characteristics of Chest Pain
)
https://beta.thecommonvein.net/wp-content/uploads/2023/06/42540c02a05.jpg https://beta.thecommonvein.net/wp-content/uploads/2024/02/71197c05a.jpg https://beta.thecommonvein.net/wp-content/uploads/2023/06/71197c08a.jpg https://beta.thecommonvein.net/wp-content/uploads/2023/06/71197c07a.jpg https://beta.thecommonvein.net/wp-content/uploads/2023/06/71197c04a.jpg https://beta.thecommonvein.net/wp-content/uploads/2023/06/71197b03p.jpg
http://thecommonvein.net/media/71197c05a.jpg http://thecommonvein.net/media/71197c07a_1.jpg http://thecommonvein.net/media/71197c08a.jpg http://thecommonvein.net/media/71197c10e.jpg http://thecommonvein.net/media/71197b03p.jpg http://thecommonvein.net/media/71197c04a.jpg
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Distribution of Somatic Nociceptors (green) and Visceral Nociceptors (pink) in the Head
71422.800b01b brain meninges pia mater = pink dura mater = green subarachnnoid space bone somatosensory receptors rich in the dura inner outer pain periosteum MRI Courtesy Ashley DAvidoff Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Distribution of Somatic Nociceptors (green) and Visceral Nociceptors (pink) in the Head
71422.800b01b brain meninges pia mater = pink dura mater = green subarachnnoid space bone somatosensory receptors rich in the dura inner outer pain periosteum MRI Courtesy Ashley DAvidoff Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] =>
[lastElementChild] =>
[childElementCount] => 0
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => 71422.800b01b brain meninges pia mater = pink dura mater = green subarachnnoid space bone somatosensory receptors rich in the dura inner outer pain periosteum MRI Courtesy Ashley DAvidoff Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => 71422.800b01b brain meninges pia mater = pink dura mater = green subarachnnoid space bone somatosensory receptors rich in the dura inner outer pain periosteum MRI Courtesy Ashley DAvidoff Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Distribution of Somatic Nociceptors (green) and Visceral Nociceptors (pink) in the Head
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Distribution of Somatic Nociceptors (green) and Visceral Nociceptors (pink) in the Head
)
https://beta.thecommonvein.net/wp-content/uploads/2023/05/71422.800b01b.jpg
http://thecommonvein.net/media/71422.800b01b_1.jpg
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Somatosensory Cortex in the Parietal Lobe
Localization and the Homunculus Man
The diagram reflects the relative functional sensory space each body part occupies in the somatosensory cortex. Those structures with high density of sensory receptors are represented by a larger size, while those with a lesser concentration of sensory apparatus shown as being “smaller” in size. Hence the mouth lips, hands feet and genitalia have a relatively large representation. The homunculus man (literally the “little man”) is the distorted figure drawn to reflect the concept of size of organ paralleling the size of the sensory innervation.
somatosensory cortex (sensory homunculus) spinothalamic tract spinal cord thalamus sensory cortex homunculus man penis clitoris genitals genitalia foot body thigh abdomen chest and face mouth eyes lips viscera somatosensory Davidoff art Copyright 2008 38610b09.46k.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Somatosensory Cortex in the Parietal Lobe
Localization and the Homunculus Man
The diagram reflects the relative functional sensory space each body part occupies in the somatosensory cortex. Those structures with high density of sensory receptors are represented by a larger size, while those with a lesser concentration of sensory apparatus shown as being “smaller” in size. Hence the mouth lips, hands feet and genitalia have a relatively large representation. The homunculus man (literally the “little man”) is the distorted figure drawn to reflect the concept of size of organ paralleling the size of the sensory innervation.
somatosensory cortex (sensory homunculus) spinothalamic tract spinal cord thalamus sensory cortex homunculus man penis clitoris genitals genitalia foot body thigh abdomen chest and face mouth eyes lips viscera somatosensory Davidoff art Copyright 2008 38610b09.46k.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The diagram reflects the relative functional sensory space each body part occupies in the somatosensory cortex. Those structures with high density of sensory receptors are represented by a larger size, while those with a lesser concentration of sensory apparatus shown as being “smaller” in size. Hence the mouth lips, hands feet and genitalia have a relatively large representation. The homunculus man (literally the “little man”) is the distorted figure drawn to reflect the concept of size of organ paralleling the size of the sensory innervation.
somatosensory cortex (sensory homunculus) spinothalamic tract spinal cord thalamus sensory cortex homunculus man penis clitoris genitals genitalia foot body thigh abdomen chest and face mouth eyes lips viscera somatosensory Davidoff art Copyright 2008 38610b09.46k.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The diagram reflects the relative functional sensory space each body part occupies in the somatosensory cortex. Those structures with high density of sensory receptors are represented by a larger size, while those with a lesser concentration of sensory apparatus shown as being “smaller” in size. Hence the mouth lips, hands feet and genitalia have a relatively large representation. The homunculus man (literally the “little man”) is the distorted figure drawn to reflect the concept of size of organ paralleling the size of the sensory innervation.
somatosensory cortex (sensory homunculus) spinothalamic tract spinal cord thalamus sensory cortex homunculus man penis clitoris genitals genitalia foot body thigh abdomen chest and face mouth eyes lips viscera somatosensory Davidoff art Copyright 2008 38610b09.46k.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Somatosensory Cortex in the Parietal Lobe
Localization and the Homunculus Man
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Somatosensory Cortex in the Parietal Lobe
Localization and the Homunculus Man
)
https://beta.thecommonvein.net/wp-content/uploads/2023/09/38610b09.46k.8s.jpg
http://thecommonvein.net/media/38610b09.46k.8s_1.jpg
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Somatosensory Cortex – Post Central Gyrus
The somatosensory cortex is overlaid in light rose pink in the diagram above and represents the most anterior structure of the parietal lobe. It lies posterior to the motor cortex (blue) which is part of the frontal lobe, behind the central sulcus and in front of the post central sulcus. It serves to perceive, localize and evaluate intensity of the pain, as well as initiate the response to the pain.
83029b01.b1.81s brain somatosensory cortex pareital lobe medial longitudinal fissure medially central sulcus anteriorly postcentral sulcus posteriorly lateral sulcus inferiorly location of primary somatosensory cortex main sensory receptive area touch. maps sensory space homunculus in this location pink = somatosensory cortex in post central gyrus blue = motor cortex The Common vein Davidoff art copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Somatosensory Cortex – Post Central Gyrus
The somatosensory cortex is overlaid in light rose pink in the diagram above and represents the most anterior structure of the parietal lobe. It lies posterior to the motor cortex (blue) which is part of the frontal lobe, behind the central sulcus and in front of the post central sulcus. It serves to perceive, localize and evaluate intensity of the pain, as well as initiate the response to the pain.
83029b01.b1.81s brain somatosensory cortex pareital lobe medial longitudinal fissure medially central sulcus anteriorly postcentral sulcus posteriorly lateral sulcus inferiorly location of primary somatosensory cortex main sensory receptive area touch. maps sensory space homunculus in this location pink = somatosensory cortex in post central gyrus blue = motor cortex The Common vein Davidoff art copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The somatosensory cortex is overlaid in light rose pink in the diagram above and represents the most anterior structure of the parietal lobe. It lies posterior to the motor cortex (blue) which is part of the frontal lobe, behind the central sulcus and in front of the post central sulcus. It serves to perceive, localize and evaluate intensity of the pain, as well as initiate the response to the pain.
83029b01.b1.81s brain somatosensory cortex pareital lobe medial longitudinal fissure medially central sulcus anteriorly postcentral sulcus posteriorly lateral sulcus inferiorly location of primary somatosensory cortex main sensory receptive area touch. maps sensory space homunculus in this location pink = somatosensory cortex in post central gyrus blue = motor cortex The Common vein Davidoff art copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The somatosensory cortex is overlaid in light rose pink in the diagram above and represents the most anterior structure of the parietal lobe. It lies posterior to the motor cortex (blue) which is part of the frontal lobe, behind the central sulcus and in front of the post central sulcus. It serves to perceive, localize and evaluate intensity of the pain, as well as initiate the response to the pain.
83029b01.b1.81s brain somatosensory cortex pareital lobe medial longitudinal fissure medially central sulcus anteriorly postcentral sulcus posteriorly lateral sulcus inferiorly location of primary somatosensory cortex main sensory receptive area touch. maps sensory space homunculus in this location pink = somatosensory cortex in post central gyrus blue = motor cortex The Common vein Davidoff art copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The Somatosensory Cortex – Post Central Gyrus
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The Somatosensory Cortex – Post Central Gyrus
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
C Fibers and the Reticular Acivating System
The image represents a coronal cut of the brain attained using T2 weighted MRI technique. It reveals a second order neuron (blue) traversing the medulla, pons, and midbrain, and in its path the C fiber component, is able to activate the RAS (pink). The stimuli reach the thalamus (orange) which not only activates the sensory cortex but other parts of the cortex as well as shown by the red lines.
C fibers pain brain reticular activating system RAS thalamus cortex medulla oblongata midbrain thalamus MRI T2 weighted Courtesy Ashley DAvidoff MD copyright 2008 77059c02.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
C Fibers and the Reticular Acivating System
The image represents a coronal cut of the brain attained using T2 weighted MRI technique. It reveals a second order neuron (blue) traversing the medulla, pons, and midbrain, and in its path the C fiber component, is able to activate the RAS (pink). The stimuli reach the thalamus (orange) which not only activates the sensory cortex but other parts of the cortex as well as shown by the red lines.
C fibers pain brain reticular activating system RAS thalamus cortex medulla oblongata midbrain thalamus MRI T2 weighted Courtesy Ashley DAvidoff MD copyright 2008 77059c02.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The image represents a coronal cut of the brain attained using T2 weighted MRI technique. It reveals a second order neuron (blue) traversing the medulla, pons, and midbrain, and in its path the C fiber component, is able to activate the RAS (pink). The stimuli reach the thalamus (orange) which not only activates the sensory cortex but other parts of the cortex as well as shown by the red lines.
C fibers pain brain reticular activating system RAS thalamus cortex medulla oblongata midbrain thalamus MRI T2 weighted Courtesy Ashley DAvidoff MD copyright 2008 77059c02.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The image represents a coronal cut of the brain attained using T2 weighted MRI technique. It reveals a second order neuron (blue) traversing the medulla, pons, and midbrain, and in its path the C fiber component, is able to activate the RAS (pink). The stimuli reach the thalamus (orange) which not only activates the sensory cortex but other parts of the cortex as well as shown by the red lines.
C fibers pain brain reticular activating system RAS thalamus cortex medulla oblongata midbrain thalamus MRI T2 weighted Courtesy Ashley DAvidoff MD copyright 2008 77059c02.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => C Fibers and the Reticular Acivating System
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => C Fibers and the Reticular Acivating System
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Second Order Neurons
Cross Over and entry into the Spinothalamic Tract
The pain fibres cross over the spinal cord via the second order neuron (blue) to the spinothalamic tract. The lateral spinothalamic tract and the anterior spinothalamic tract There are two parts to the anterolateral spinothalamic tract. The lateral spinothalamic tract (darker blue) carries the fibers for pain and temperature sensations and the anterior spinothalamic tract (light blue) carries sensation of simple touch.
orange = sensory nerve carrying stimuli from periphery
blue = anterolateral spinothalamic tract
dark blue = lateral spinothalamic tract
light blue = anterior spinothalamic tract
Davidoff art Courtesy Ashley Davidoff MD copyright 2008 83067b05b05.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Second Order Neurons
Cross Over and entry into the Spinothalamic Tract
The pain fibres cross over the spinal cord via the second order neuron (blue) to the spinothalamic tract. The lateral spinothalamic tract and the anterior spinothalamic tract There are two parts to the anterolateral spinothalamic tract. The lateral spinothalamic tract (darker blue) carries the fibers for pain and temperature sensations and the anterior spinothalamic tract (light blue) carries sensation of simple touch.
orange = sensory nerve carrying stimuli from periphery
blue = anterolateral spinothalamic tract
dark blue = lateral spinothalamic tract
light blue = anterior spinothalamic tract
Davidoff art Courtesy Ashley Davidoff MD copyright 2008 83067b05b05.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 5
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The pain fibres cross over the spinal cord via the second order neuron (blue) to the spinothalamic tract. The lateral spinothalamic tract and the anterior spinothalamic tract There are two parts to the anterolateral spinothalamic tract. The lateral spinothalamic tract (darker blue) carries the fibers for pain and temperature sensations and the anterior spinothalamic tract (light blue) carries sensation of simple touch.
orange = sensory nerve carrying stimuli from periphery
blue = anterolateral spinothalamic tract
dark blue = lateral spinothalamic tract
light blue = anterior spinothalamic tract
Davidoff art Courtesy Ashley Davidoff MD copyright 2008 83067b05b05.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The pain fibres cross over the spinal cord via the second order neuron (blue) to the spinothalamic tract. The lateral spinothalamic tract and the anterior spinothalamic tract There are two parts to the anterolateral spinothalamic tract. The lateral spinothalamic tract (darker blue) carries the fibers for pain and temperature sensations and the anterior spinothalamic tract (light blue) carries sensation of simple touch.
orange = sensory nerve carrying stimuli from periphery
blue = anterolateral spinothalamic tract
dark blue = lateral spinothalamic tract
light blue = anterior spinothalamic tract
Davidoff art Courtesy Ashley Davidoff MD copyright 2008 83067b05b05.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Second Order Neurons
Cross Over and entry into the Spinothalamic Tract
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Second Order Neurons
Cross Over and entry into the Spinothalamic Tract
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Fibers involved in Gate Control Mechanism
This diagram shows the three types of receptors and fibers that transmit impulses related directly and indirectly to pain . The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, measuring between .4 to 1.2 micrometers, and conducts the impulse at about 2m/s. The second neuron is the A delta fiber and it responds to the pricking or sharp sensation that is first felt and reacted to. It is weakly myelinated and is about 2-6 micro meters thick, and conducts the stimulus with a velocity of between 15-30 meters per second. The last fiber is the A beta fiber and it is responsible for the pressure component which indirectly affects response to pain by affecting the gate mechanism of pain. It is greater than 10 microns thick due to heavier myelination and conducts impulses at 30-100meters per second
fiber neuron long peripheral process short central process ganglion cell ganglion body nerve sensory nerve dorsal ganglion dorsal column synapse Davidoff art Courtesy Ashley Davidoff MD Copyright 2008 83066c05b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Fibers involved in Gate Control Mechanism
This diagram shows the three types of receptors and fibers that transmit impulses related directly and indirectly to pain . The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, measuring between .4 to 1.2 micrometers, and conducts the impulse at about 2m/s. The second neuron is the A delta fiber and it responds to the pricking or sharp sensation that is first felt and reacted to. It is weakly myelinated and is about 2-6 micro meters thick, and conducts the stimulus with a velocity of between 15-30 meters per second. The last fiber is the A beta fiber and it is responsible for the pressure component which indirectly affects response to pain by affecting the gate mechanism of pain. It is greater than 10 microns thick due to heavier myelination and conducts impulses at 30-100meters per second
fiber neuron long peripheral process short central process ganglion cell ganglion body nerve sensory nerve dorsal ganglion dorsal column synapse Davidoff art Courtesy Ashley Davidoff MD Copyright 2008 83066c05b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => This diagram shows the three types of receptors and fibers that transmit impulses related directly and indirectly to pain . The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, measuring between .4 to 1.2 micrometers, and conducts the impulse at about 2m/s. The second neuron is the A delta fiber and it responds to the pricking or sharp sensation that is first felt and reacted to. It is weakly myelinated and is about 2-6 micro meters thick, and conducts the stimulus with a velocity of between 15-30 meters per second. The last fiber is the A beta fiber and it is responsible for the pressure component which indirectly affects response to pain by affecting the gate mechanism of pain. It is greater than 10 microns thick due to heavier myelination and conducts impulses at 30-100meters per second
fiber neuron long peripheral process short central process ganglion cell ganglion body nerve sensory nerve dorsal ganglion dorsal column synapse Davidoff art Courtesy Ashley Davidoff MD Copyright 2008 83066c05b.8s
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => This diagram shows the three types of receptors and fibers that transmit impulses related directly and indirectly to pain . The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, measuring between .4 to 1.2 micrometers, and conducts the impulse at about 2m/s. The second neuron is the A delta fiber and it responds to the pricking or sharp sensation that is first felt and reacted to. It is weakly myelinated and is about 2-6 micro meters thick, and conducts the stimulus with a velocity of between 15-30 meters per second. The last fiber is the A beta fiber and it is responsible for the pressure component which indirectly affects response to pain by affecting the gate mechanism of pain. It is greater than 10 microns thick due to heavier myelination and conducts impulses at 30-100meters per second
fiber neuron long peripheral process short central process ganglion cell ganglion body nerve sensory nerve dorsal ganglion dorsal column synapse Davidoff art Courtesy Ashley Davidoff MD Copyright 2008 83066c05b.8s
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Fibers involved in Gate Control Mechanism
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Fibers involved in Gate Control Mechanism
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
From the Spinal Cord to the Brain and Perception of the Pain
The Three Orders of Neurons
The stimulus is converted into an electrical impulse which is taken by a first order sensory nerve (orange) to the spinal cord (dorsal root ) which in turn transports the impulse via second order neuron (dark blue and light blue) to the thalamus,. The third order neuron (pink) transports the impulse to the somatosensory cortex.
77533b06d06.8s pain skin joint muscle nociceptors dorsal root ganglion spinal cord brain thalamus sensory cortex somatosensory cortex postcentral gyrus pain perception localization perceive Davidoff art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
From the Spinal Cord to the Brain and Perception of the Pain
The Three Orders of Neurons
The stimulus is converted into an electrical impulse which is taken by a first order sensory nerve (orange) to the spinal cord (dorsal root ) which in turn transports the impulse via second order neuron (dark blue and light blue) to the thalamus,. The third order neuron (pink) transports the impulse to the somatosensory cortex.
77533b06d06.8s pain skin joint muscle nociceptors dorsal root ganglion spinal cord brain thalamus sensory cortex somatosensory cortex postcentral gyrus pain perception localization perceive Davidoff art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The stimulus is converted into an electrical impulse which is taken by a first order sensory nerve (orange) to the spinal cord (dorsal root ) which in turn transports the impulse via second order neuron (dark blue and light blue) to the thalamus,. The third order neuron (pink) transports the impulse to the somatosensory cortex.
77533b06d06.8s pain skin joint muscle nociceptors dorsal root ganglion spinal cord brain thalamus sensory cortex somatosensory cortex postcentral gyrus pain perception localization perceive Davidoff art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The stimulus is converted into an electrical impulse which is taken by a first order sensory nerve (orange) to the spinal cord (dorsal root ) which in turn transports the impulse via second order neuron (dark blue and light blue) to the thalamus,. The third order neuron (pink) transports the impulse to the somatosensory cortex.
77533b06d06.8s pain skin joint muscle nociceptors dorsal root ganglion spinal cord brain thalamus sensory cortex somatosensory cortex postcentral gyrus pain perception localization perceive Davidoff art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => From the Spinal Cord to the Brain and Perception of the Pain
The Three Orders of Neurons
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => From the Spinal Cord to the Brain and Perception of the Pain
The Three Orders of Neurons
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
The Dorsal Root Ganglion of the Afferent Neurons
The dorsal root ganglion is a focal accumulation of the first order nerve cells of the sensory component of the peripheral nerve. (orange) It is situated in the neural foramen of the vertebral body. The central process emanates from the ganglion cell and ends in the dorsal horn.
83067b03b04.8s peripheral nerve sensory nerve motor nerve dorsal root ganglion dorsal horn pain pathway spinal cord Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
The Dorsal Root Ganglion of the Afferent Neurons
The dorsal root ganglion is a focal accumulation of the first order nerve cells of the sensory component of the peripheral nerve. (orange) It is situated in the neural foramen of the vertebral body. The central process emanates from the ganglion cell and ends in the dorsal horn.
83067b03b04.8s peripheral nerve sensory nerve motor nerve dorsal root ganglion dorsal horn pain pathway spinal cord Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The dorsal root ganglion is a focal accumulation of the first order nerve cells of the sensory component of the peripheral nerve. (orange) It is situated in the neural foramen of the vertebral body. The central process emanates from the ganglion cell and ends in the dorsal horn.
83067b03b04.8s peripheral nerve sensory nerve motor nerve dorsal root ganglion dorsal horn pain pathway spinal cord Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The dorsal root ganglion is a focal accumulation of the first order nerve cells of the sensory component of the peripheral nerve. (orange) It is situated in the neural foramen of the vertebral body. The central process emanates from the ganglion cell and ends in the dorsal horn.
83067b03b04.8s peripheral nerve sensory nerve motor nerve dorsal root ganglion dorsal horn pain pathway spinal cord Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The Dorsal Root Ganglion of the Afferent Neurons
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The Dorsal Root Ganglion of the Afferent Neurons
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Types of Receptors Subtending the A delta Fibers and C Fibers
The diagram shows sensory stimuli including sharp pressure, extreme heat and cold as well as chemical, stimulating the free nerve endings of the nociceptors that are linked to the myelinated A delta fiber , and non myelinated C fiber. The myelinated fiber will conduct the impulse between 3 and 15 times faster than the non myelinated fiber.
87559pc08b03.8s
pain free nerve ending nociceptor A delta fibres C fibres heat cold pressure mechanical prick afferent somatosensory nerves somatic anatomy normal Davidoff art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Types of Receptors Subtending the A delta Fibers and C Fibers
The diagram shows sensory stimuli including sharp pressure, extreme heat and cold as well as chemical, stimulating the free nerve endings of the nociceptors that are linked to the myelinated A delta fiber , and non myelinated C fiber. The myelinated fiber will conduct the impulse between 3 and 15 times faster than the non myelinated fiber.
87559pc08b03.8s
pain free nerve ending nociceptor A delta fibres C fibres heat cold pressure mechanical prick afferent somatosensory nerves somatic anatomy normal Davidoff art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 2
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The diagram shows sensory stimuli including sharp pressure, extreme heat and cold as well as chemical, stimulating the free nerve endings of the nociceptors that are linked to the myelinated A delta fiber , and non myelinated C fiber. The myelinated fiber will conduct the impulse between 3 and 15 times faster than the non myelinated fiber.
87559pc08b03.8s
pain free nerve ending nociceptor A delta fibres C fibres heat cold pressure mechanical prick afferent somatosensory nerves somatic anatomy normal Davidoff art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The diagram shows sensory stimuli including sharp pressure, extreme heat and cold as well as chemical, stimulating the free nerve endings of the nociceptors that are linked to the myelinated A delta fiber , and non myelinated C fiber. The myelinated fiber will conduct the impulse between 3 and 15 times faster than the non myelinated fiber.
87559pc08b03.8s
pain free nerve ending nociceptor A delta fibres C fibres heat cold pressure mechanical prick afferent somatosensory nerves somatic anatomy normal Davidoff art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Types of Receptors Subtending the A delta Fibers and C Fibers
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Types of Receptors Subtending the A delta Fibers and C Fibers
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
C Fiber
The C fiber are small in size, are non myelinated, and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
83066c02c.8s This diagram shows the two types of receptors and fibers that transmit the pain impulse. The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 2m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
C Fiber
The C fiber are small in size, are non myelinated, and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
83066c02c.8s This diagram shows the two types of receptors and fibers that transmit the pain impulse. The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 2m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => The C fiber are small in size, are non myelinated, and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
83066c02c.8s This diagram shows the two types of receptors and fibers that transmit the pain impulse. The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 2m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => The C fiber are small in size, are non myelinated, and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
83066c02c.8s This diagram shows the two types of receptors and fibers that transmit the pain impulse. The upper fiber is called the C fiber and it is non myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 2m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => C Fiber
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => C Fiber
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
A Delta Fiber
The A delta fiber consist of free nerve endings, are of intermediate size, are minimally myelinated (yellow sheath) and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
883066c02bd.8s This diagram shows A delta fiber whic is minimally myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 15-30m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
A Delta Fiber
The A delta fiber consist of free nerve endings, are of intermediate size, are minimally myelinated (yellow sheath) and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
883066c02bd.8s This diagram shows A delta fiber whic is minimally myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 15-30m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
A Delta Fiber
The A delta fiber consist of free nerve endings, are of intermediate size, are minimally myelinated (yellow sheath) and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
883066c02bd.8s This diagram shows A delta fiber whic is minimally myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 15-30m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
A Delta Fiber
The A delta fiber consist of free nerve endings, are of intermediate size, are minimally myelinated (yellow sheath) and consist of a long peripheral process and a short central process, which connects the neuron to the dorsal horn in the gray matter of the spinal cord.
883066c02bd.8s This diagram shows A delta fiber whic is minimally myelinated, consists of the receptors in the top left hand corner that when stimulated transmit the impulse via a long afferent neuron to the cell body lying alongside the spinal column. This fiber is relatively thin, and conducts the impulse at about 15-30m/s. nerve sensory system nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => table
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] => (object value omitted)
[nextElementSibling] => (object value omitted)
[nodeName] => table
[nodeValue] =>
Nociceptors and their Pathway to the Spinal Cord
The sensory receptors of the nociceptors are found in the tissues peripherally (top left) and are connected by a long fiber that transmits the impulse to the ganglion cell that lies in the dorsal ganglion in the neural canal alongside the spinal cord.
83066b09.8sb nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => table
[baseURI] =>
[textContent] =>
Nociceptors and their Pathway to the Spinal Cord
The sensory receptors of the nociceptors are found in the tissues peripherally (top left) and are connected by a long fiber that transmits the impulse to the ganglion cell that lies in the dorsal ganglion in the neural canal alongside the spinal cord.
83066b09.8sb nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 3
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] => Nociceptors and their Pathway to the Spinal Cord
The sensory receptors of the nociceptors are found in the tissues peripherally (top left) and are connected by a long fiber that transmits the impulse to the ganglion cell that lies in the dorsal ganglion in the neural canal alongside the spinal cord.
83066b09.8sb nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] => Nociceptors and their Pathway to the Spinal Cord
The sensory receptors of the nociceptors are found in the tissues peripherally (top left) and are connected by a long fiber that transmits the impulse to the ganglion cell that lies in the dorsal ganglion in the neural canal alongside the spinal cord.
83066b09.8sb nociceptor A delta fober C fiber pain stimulus neuron receptor afferent pathway sensory dorsal ganglion dorsal column sensory pathway Davidoff Art Copyright 2008
)
DOMElement Object
(
[schemaTypeInfo] =>
[tagName] => td
[firstElementChild] => (object value omitted)
[lastElementChild] => (object value omitted)
[childElementCount] => 1
[previousElementSibling] =>
[nextElementSibling] =>
[nodeName] => td
[nodeValue] =>
[nodeType] => 1
[parentNode] => (object value omitted)
[childNodes] => (object value omitted)
[firstChild] => (object value omitted)
[lastChild] => (object value omitted)
[previousSibling] => (object value omitted)
[nextSibling] => (object value omitted)
[attributes] => (object value omitted)
[ownerDocument] => (object value omitted)
[namespaceURI] =>
[prefix] =>
[localName] => td
[baseURI] =>
[textContent] =>
)
 Acute Pelvic Pain Awakening the Patient
Acute Pelvic Pain Awakening the Patient