Treatment and Management Considerations in Pain
Clinical Strategies
Copyright 2008
James Armstrong PA
Ashley Davidoff MD
Basis of Pain Therapy
A pain experience for an equal degree of injury will vary among patients. So too will the treatments they receive. In more pedestrian cases of pain, simply removing the offending agent will suffice. However in many instances this simple and logical process does not suffice and modern medicine has developed a science of pain management in order to relieve patients the burden of pain. The science of pain is based on many of the principles discussed in the first modules relating to the anatomy, physiology and diseases associated with pain. We will review these principles briefly in order to frame the manner in which pain management is utilized.
Structural considerations
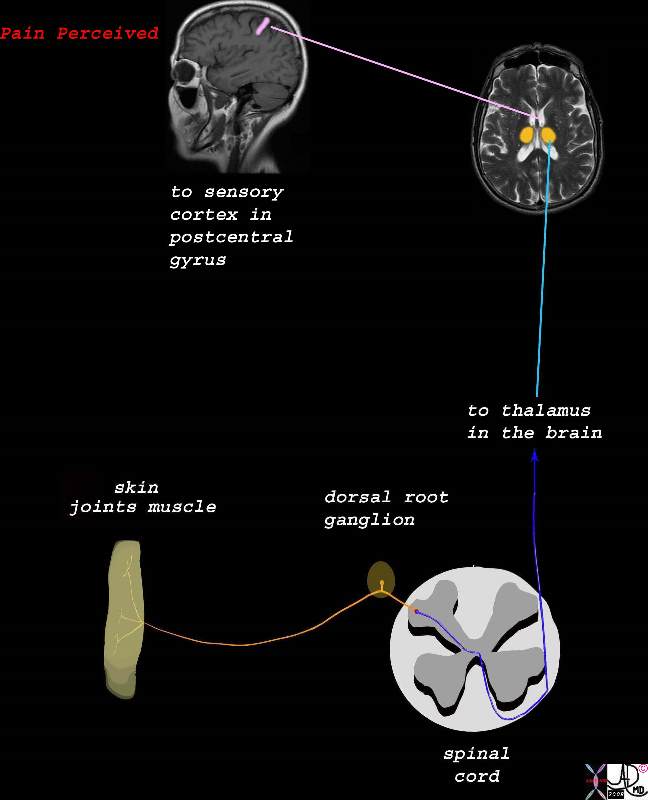
There are many orders of structural complexity in the pain process, but in general we can view pain stimulus as being received and transported by first order neurons to the spinal cord, which synapse with second order neurons in the dorsal horn. The second order neurons transport the pain to the thalamus. The third order neuron takes the pain stimulus to various parts of the brain where perception takes place and a variety of reactions to the pain are expressed.
Functional Considerations
We reviewed the many mechanisms by which the pain experience was modulated and how the body modified and individualized the pain experience. The electrical mechanisms and neurotransmitters are numerous and complex, but this allows the application of a variety of techniques and options to deal with pain
Consideration in Disease
Lastly we reviewed how disease processes modified the threshold of pain, and in particular how inflammation via a variety of mechanisms lowered the threshold to pain. Some pain is best treated by targetting and reducing the inflammatory response
Considerations in treatment
Based on the science, it becomes intuitive to aim treatment at the brain, spinal cord, or at peripheral nerves and their ganglia.
Pain control can be non specifically directed at the nervous system resulting in the loss of a variety of sensations including pain. This is called anesthesia which by definition means loss of sensation. As a result of the anesthesia, whether administered locally or generally, pain is controlled, and in the case of general anesthesia there is associated loss of conciousness. It is usually used preemptively for surgical intervention. Analgesia is another variant of pain control, usually used after the injury or disease has occurred and without altering conciousness. There are many agents specfically designed to treat pain mechanisms.
Anesthesia
Local and regional anesthesia aim therapy at the nerves and ganglia.The anesthetic in these instances is injected locally and directly into the tissue or nerve involved. Spinal anesthesia (subarachnoid anesthesia) and epidural anesthesia are directed at the nerve-spinal cord interface. The anesthesia in these instances are administered locally and directly by a needle dierected into the spaces surrounding the cord. Anesthetic agents administered intravenously or via the airways may take the form of general anesthesia, deep sedation, concious sedation and minimal sedation. These agents that act on the brain alter the level of conciousness.
However when dealing with pain in the context of disease and long term relief, we are in general dealing with the field of analgesia, since anesthesia is in general short lived.
Analgesia
Briefly, the pain signal is initiated by a noxious insult or process causing cellular depolarization of peripheral nociceptors. The signal then propagates as a tsunami of depolarization along the process of a neuron. With the help of neurotransmitters, the wavefront crosses neurosynaptic bridges transmitting over additional neurons as it travels centrally to the dorsal horn of the spine. At this level, the signal receives input from other biochemical and mechanical influences. The signal then travels up the spinal cord to the brain where it is dispersed among the anatomical centers that comprise the limbic system. Here begins a competitive interaction of pain promotion by biogenic amine neurotransmitters (acetylcholine and norepinephrine) secreted from the hypothalamus and reticular acitvating system (RAS) versus pain suppression by indolamines (serotonin), dopamine and morphine-like B-endorphin peptides called enkephalins from the periventricular gray matter. The limbic system thus modifies then finalizes the perception of the pain signal and incorporates its interpretation with emotion and behavior to elicit a response. Analgesics provide a pharmacological means of intervening upon the different components of this pain cascade.
Modifying Inflammation
Parallel to the pain pathways, tissue injury promotes the synthesis of prostaglandins peripherally and centrally. These compounds play many vital roles in the body but are the main biochemical component of inflammation. As described in the functional pain module, inflammation is the primary tissue response to disease or injury. This response however is mediated by the local release of prostaglandins. Their presence promotes vasodilation and edema. The consequence of this tissue infiltration is further stretching and stimulation of peripheral pain receptors and increased pain intensity. Analgesics provide a pharmacological means of intervening upon the various components of this pain cascade.
Centrally, prostaglandin synthesis is intimately tied to the emotional response to pain as the associated anxiety promotes its production from the hypothalmus. These substances also intensify pain in the limbic system especially in cases of repeated insult or injury.
The first therapeutic approach to somatic pain from inflammation is to immobilize the area if possible and to apply cold compression and elevation. This therapy provides mechanical modification to the pain receptors by minimizing vasodilation and promoting venous return thereby decreasing edema. In addition, nociceptor activity is directly down regulated by the local hypothermia of an ice bag.
For visceral or intense somatic pain, drugs with anti-inflammatory activity can significantly improve the experience. There are four main groups and six sub-groups of these agents, each with its own unique effect on inflammation.
Corticosteroids are endogenous compounds secreted from the adrenal glands. Their synthetic versions are effective analgesics that regulate inflammation directly by downregulating the immune response and indirectly by decreasing the body’s overall stress response to pain. They are also useful adjuncts to other analgesics by their positive effects on mood and appetite. Corticosteroids can be administered orally or injected locally in combination with other pain relievers especially in cases of chronic joint pain. Systemic use of these agents requires caution as their chronic use creates a negative feedback loop to the adrenal glands thereby suppressing endogenous corticosteroid production. There are a number of side effects with these agents and they include gastrointestinal irritation, osteopenia, leukocytosis and immunosuppression.
Salicylates (e.g. aspirin) inhibit prostaglandin synthesis both in the CNS and in the periphery. Aspirin remains the oldest and cheapest salicylate analgesic available and its use remains high due to its additional anti-platelet, anti-cancer and antipyretic properties. Unfortunately aspirin’s antiprostaglandin effects are universal as prostaglandins that are protective to the mucosal lining of the stomach are also inhibited. The major side effect of this agent therefore is gastritis and potentially life threatening gastrointestinal (GI) bleeding.
Acetaminophen (e.g. Tylenol®) is an effective antipyretic and analgesic agent that primarily inhibits prostaglandin activity within the CNS but not peripherally. Acetaminophen therefore does not reduce inflammation at the site of injury but is an effective pain reliever nontheless. Higher doses of this agent can be toxic to the liver and kidneys.
Nonsteroidal anti-inflammatory drugs (NSAIDS eg aspirin ibuprofen and naloxen) are a diverse class of drugs that combine anti-inflammatory, analgesic and and antipyretic effects with varied potencies and mechanisms of action on prostaglandin activity.
Proprionic acids (ibuprofen, fenoprofen, proxen, fenbufen, ketoprofen, birbuofen proquazone and oxaprozin) are the most popular nonsteroidal anti-inflammatory preparations. At lower doses, these compounds exhibit adequate analgesic activity especially in the CNS. However, their “forte” of potent anti-inflammatory activity is not experienced until higher doses are consumed. Again, GI side effects from prostaglandin inhibition are a correlative risk. Often, concomitant use of acid reducing agents and/or limitations on therapy duration are recommended.
Indoleacetic acid (indomethacin) exhibit potent and nonspecific prostaglandin inhibition and are effective for cases of acute somatic pain especially in rheumatoid arthritis exacerbations or conditions of inflamed visceral linings. For example, effective relief of pleuritic chest pain from pericarditis is often responsive to this drug. GI irritation is unfortunately fairly common with this agent and therapy should thus be short-lived.
Idene acetic acids (sulindac, zomepirac), phenylacetic acid (diclofenac) and pyrrole acetic acid (tolmetin) are chemically related to indomethacin and thus have similar global antiprostaglandin properties. Their efficacy is thus tempered by the same potential toxic GI side effects.
Benzothiazine (piroxicam) is a stable compound with a longer half-life and thus anti-inflammatory effects over a 24 hour period.
Anthranilic acid (meclofenamate) is a unique anti-inflammatory analgesic in that it competitively inhibits prostaglandin receptors in the periphery.
Pyrazolines (phenylbutazone, azapropazone) are primarily anti-inflammatory with only minimal analgesic activity rendering these agents specific to only a few pain related entities. Toxicity from these drugs includes hematological abnormalities and edema. Duration of administration is therefore short-term except for the treatment of ankylosing spondylitis.
Modifying Pain Perception
The key to analgesic efficacy within the pain centers of the brain is the ability to offset the balance of pain stimulation and suppression in favor of the latter. The more intense or prolonged the pain experience, the lower the pain threshold and the higher the sleep deprivation, anxiety and depression. Analgesic agents that augment the pain suppressing neurotransmitters serotonin, dopamine, and catecholamines can effectively change the perception of pain. The most effective way to increase neurotransmitter levels is to inhibit their synaptic reuptake. When a neuronal signal has crossed the synapse, facilitated by the presence of neurotransmitters, these compounds are quickly restored in the neuron via cyclic adenosine monophosphate (cAMP). By competing with the receptors that promote this peptide transporter, the reuptake mechanism of serotonin and acetylcholine can be inhibited and the pain experience thus modified.
Antidepressants
Tricyclic antidepressant medications are essentially designed to suppress the synthesis of indolamines and catecholamines in the CNS to improve mood and decrease anxiety. A second function of these drugs is to provide cAMP inhibition and thus increased synaptic concentrations of serotoin and acetylcholine leading to better regulation of sleep patterns and an increase in pain threshold. Their third property is to increase concentrations of enkephalins in the periventricular areas of the brain and provide a morphine-like effect.
Another class of antidepressants known as selective serotonin reuptake inhibitors (SSRIs) enhance serotonin levels even more than the tricylics because they can specifically target the presynaptic serotonin mechanism. Post-synaptic inhibition can increase catecholamine levels and offset the analgesic result.
Anxiolytics
This group of compounds known commonly as sedative tranquilizers exhibit various mechanisms of action that mitigate the anxiety that complicates pain. The first of these agents are the phenothiazines. They have direct and indirect analgesic effects and are indicated for chronic pain. The second group is the benzodiazepines, indicated only for acute pain therapy due to their long term addictive hypnotic and withdrawal properties. Benzodiazepines are considered indirect analgesics because they only treat the sequelae associated with pain. Two other categories of drugs with anxiolytic effects are azapirones and hydroxyzine, an antihistamine.
Phenothiazines
The phenothiazine class (fluphenazine, trifluoperazine, and perphenazine) have a minimal net analgesic effect as they block both postsynaptic dopamine, an analgesic and norepinephrine, a pain promoter. They do however inhibit the enzymatic degradation of enkephalins thus increasing their morphine-like effects. These agents also block post synaptic noradrenergic receptors thereby lessening the pain intensifying effect of the sympathetic nervous system. Finally, when used in combination with tricyclic antidepressants, phenothiazines exhibit their strongest influence on pain by decreasing anxiety and promoting sleep. Medications from this class are relatively free of addictive properties and are therefore frequently an important component of the drug regimen for chronic pain patients.
Benzodiazepines
The benzodiazepines, including midazolam (Versed), alprazolam (Xanax), flurazepam (Dalmane), diazepam (Valium), chlordiazepoxide (Librium), oxazepam (Serax), chlorazepate (Tranzene), lorazepam (Ativan) and clinidium bromide (Librax) aid in the treatment of pain in two indirect ways. First, they act on specific receptors in the CNS to reduce anxiety. Second, they competitivey inhibit glycine receptors at the level of the spinal cord to reduce spasm, a major contributor to somatic pain. These drugs therefore have some use for pain in the acute setting. However, there are many potential problems with this drug class, especially in the treatment of chronic pain.
We know that serotonin is an effective pain reducing neurotransmitter in the CNS. Benzodiazepines actually lower serotonin levels thereby decreasing its pain dampening effects and the overall pain threshold. This is achieved via a third mechanism of action, inhibiting gamma aminobutyric acid (GABA) receptors that normally facilitate the presynaptic release of serotonin. These agents also reduce rapid eye movement (REM) sleep, another serotonin building process. Benzodiazepines are unfortunately also highly addictive for their sedative effects. In addition, their activity on GABA receptors can be anticonvulsive, similar to alcohol or phenobarbitol. Consequently, sudden withdrawal from these agents can cause seizures.
Hydroxyzine (Vistaril) is an antihistamine that plays an important role in reducing acute anxiety associated with chronic pain flare-ups. More importantly however, this special antihistamine reduces the experience of drug withdrawal especially from benzodiazepines by rebalancing REM sleep patterns.
The azapirones include buspirone, gepirone and ipsapirone and are another class of anxiolytics that potentiate the expression of the serotonin specific 5-HTIA receptor. These drugs therefore contribute to reducing pain anxiety and also exhibit antidepressive effects.
Muscle Relaxants
Muscle spasm is a mechanical pain response and is often a major contributor to an already unpleasant experience. Muscle relaxants can work centrally in the spinal cord or peripherally. The central agents like baclofen in the treatment of multiple sclerosis for example act similarly to the benzodiazepines inhibiting glycine and GABA receptors and reducing the involuntary spasms associated with this disease. The most common peripheral muscle relaxant prescribed is cyclobenzaprine (Flexeril). Its mechanism of action is not well understood but is related to increasing local levels of norepinephrine within the muscle spindle. This agent however has some central effects as its common side effect include drowsiness, blurry vision and depression.
Anticonvulsants
Carbamzepine (Tegretol) in the treatment of trigeminal neuralgia and valproic acid and gabapentin (Nerontin) for other post-therapeutic neuralgias are effective suppressors of non-nocipetor neuronal pain. Their mechanisms are not well understood. Neurontin is also very effective for diabetic neuropathy.
Special Considerations
Other agents effective for specific pain syndromes include propanolol for chronic vascular headaches, phentolamine for reflex sympathetic dystrophy, mexilitene for diabetic neuropathy and L-dopa in the treatment of bone metastases. Again, their mechanisms of action are poorly understood and many of the therapeutic indications lack prospective randomized trials.
Modifying the CNS – Opioids
First and Second Order Neuron Effects
Opioid medications include morphine and several other synthetic and naturally occurring narcotics. These analgesics have a far different pharmacologic mechanism for reducing pain than previously mentioned. Opioids bind specifically to a heterogeneous group of receptors in the CNS but cast their analgesic effects both centrally and peripherally on nociceptive first and second order neurons.
Peripherally, opioid receptors exist in their highest concentrations within the presynaptic terminals of first order neurons within the substantia gelatinosa in the dorsal horn of the spinal cord. They are also plentiful on the post-synaptic dendrites of the second order and inter-neurons that modify the pain signal during spinothalamic transmission. Via their competitive affinity for the presynaptic receptors, opioids inhibit the release of substance P and glutamate into the synapse of afferent neurons thus mitigating the action potential of the pain signal. This phenomenon occurs with nociceptive, sympathetic and motor neurons.
Centrally, opioids dampen the limbic system response and the emotion, anxiety and sleeplessness of pain. They also activate descending pain inhibitory pathways from the medulla and periaqueductal gray matter which attenuates ascending nociceptive transmission. Lastly, opioids can provide direct inhibition the first order neurons in the spinal cord.
Opioid receptor binding is a steric specific phenomenon. There are multiple sub-types of these receptors and a variety of molecular structures comprising synthetic and natural opioid compounds. Once bound, the receptor/opioid complex undergoes a morphological change that minimizes potassium and sodium ion channels and therefore directly inhibits conduction of the pain impulse. At the nociceptor level, the action potential is blunted in amplitude and duration thereby reducing calcium ion influx and the subsequent release of substance P and glutamate.
Opioid Agonism and Antagonism
The physical and chemical properties of the opioids determine the speed of onset, duration and potency of the analgesic effect. Some drugs of this class may completely override the pain signal while others may require adjuvant anti-inflammatory or anesthetic agents. Pure agonists have the strongest affinity for receptors that influence the presynaptic terminal to produce a purely opioid effect. Morphine, fentanyl and hydromorphone are the naturally occurring pure agonists. Codeine, oxycodone (1 ½ times the potency of morphine) and hydrocodone are synthetic examples of pure agonists. Severe acute pain and chronic cancer pain are the afflictions most commonly treated with these analgesics.
Opioid antagonist activity in contrast is a property of the drug nalaxone, used to reverse the narcotic effect of morphine-like drugs in overdose situations. Nalaxone has no intrinsic anti-analgesic activity but competitively displaces the opioid from its receptor. It is commonly available in urban emergency rooms for obtunded addicts.
Agonist/antagonists have hybrid binding owing to the steric heterogeneity of the opioid receptors. Pentazocine, butorphanol, nalbuphine, and buprenorphine define this class. Their analgesic properties have a ceiling that limits their use in cancer pain for example. Higher doses of this opioid class may also have psychogenic effects.
Opioid Side Effects
Opioid use requires careful consideration and review of a patient?s pain scenario due to their potentially dangerous side effects and the risk of tolerance and addiction.
Unfortunately, opioid receptors exist in the GI tract and their suppression can inhibit motility leading to the most common side effects ranging from constipation to nausea and vomiting. When severe enough, bowel obstruction may occur. Opioid receptors also exist in the respiratory centers of the brain and opioid analgesics may consequently inhibit proper respiratory drive, especially in high doses. Other negative side effects exist and should be reviewed prior to their use. All opioid analgesics must be administered carefully so as to maximize safety.
Tolerance to opioids is a phenomenon where a patient chronically taking an opioid gradually loses the ability to obtain an analgesic effect. There are two tolerance classifications. The first is behavioral tolerance. At low or less frequent dosing intervals, this phenomenon usually occurs as a consequence of habitual associations. The tolerance is more of a learned behavior. Pharmacological tolerance on the other hand is seen in higher and more consistent dose intervals. This is truly a drug interaction where physiological adaptations of the opioid receptors occur. When withdrawal of the opioid causes stressful physiological sequelae and craving, the situation may be described as physical dependence. The point where drug seeking behavior is constant and life altering is known as addiction. The patient is overwhelmed by the need to fulfill the analgesic effect. Drug addiction is a reality in any clinical setting where chronic pain therapy is administered. Prompt recognition and communication among colleagues, patients and their families is essential in getting those who are within its grasp into a harmonious balance of pain control and independence.
Administering Opioids
Intraspinal
Opioids have a wide range of effects on neuronal excitability predicated on their preparation and the route of administration. Their bioavailability increases their potency in correlation to the level of invasiveness of drug delivery. One of the most common therapies for severe acute pain, especially with obstetric labor is intraspinal therapy. The opioid is delivered via a small catheter placed in the spinal or epidural space within the spinal column. Chronic back pain treatment can be administered with the use of an implanted spinal infusion pump. The advantages of intraspinal therapy is more complete analgesia at lower doses of opioid and fewer of the sedating or GI side effects of the drug.
Tramadol is a synthetic opioid analog that achieves central analgesic effects without binding to opioid receptors. It is the only one of its kind and is indicated for many pain syndromes, especially neuralgias. Although, it may also cause GI and respiratory side effects, they are less severe and without the hazards of drug dependence.
Local Injection
Opioids are often formulated with an anesthetic and/or steroid for injection or infusion to a body area for chronic pain. These cocktails often provide effective analgesia especially in inflammatory or neuronal pain syndromes. The honeymoon of relief however usually has an endpoint and repeated therapeutic injections are common.
Injectable analgesic preparations are also useful as regional blocks where a nerve plexus or ganglion is causing chronic pain. Stellate ganglion block for cervical radiculopathy is a good example. Nerve block therapy may also provide motor dysfunction to areas of that cause visceral pain. Chronic pelvic pain can improve with hypogastric plexus block and celiac plexus block is sometimes useful for chronic abdominal pain. There are risks to these procedures including dysfunction of adjacent organs or systems.
The use of these techniques especially for the treatment of chronic pain has generated an entire field of medicine due to its prevalence in society and the inherent risks of addiction. The evolution of anesthesiologist directed pain clinics where these procedures can be performed safely has dramatically improved the support network for chronic pain patients.
Surgical and Interventional Techniques
Surgical treatment is commonly indicated to relieve pain. Decompression of localized skin abscesses bring rapid relief. Surgical procedures that reduce or treat distension or obstruction, always bring pain relief. The treatment may be as simple as placing a nasogastric tube to reduce the distension of bowel obstruction, but may require more complex surgery such as removing a tumor that has created the bowel obstruction. Sometimes the relief comes from treating disease that has its origins in obstruction as well as inflammation and or infection. Acute cholecystitis and appendicitis are such instances. The organ is initally distended by an obstructive cause and then it becomes secondarily infected. Removing the gallbladder or appendix treats the root cause of pain and then the healing process will need medical support for pain relief. Ischemia is another cause of pain that can be treated by the surgeon or minimally invasive interventionalist. Stenosis of an artery will cause ischemia and pain such as in angina. A few treatment options are available including angioplasty, stent placement, or bypass each of which will restore normal flow and treat the pain. Infarction is also painful, and in the case of bowel infarction the injured bowel has to be surgically removed. The pain from trauma is multifaceted but includes somatic and visceral pain from tissue injury, and continued tension from swelling, hematoma and malaligned bones for example. With accurate diagnosis, the approach to trauma and treatment of pain in these patients is also multifaceted .
Conclusion
The treatment of pain is a challenge to all caregivers. Knowledge of the pain pathways, their physiology, the pathophysiology of pain and the treatment options is necessary for those involved in the diagnosis and treatment of pain.
Treatment sometimes involves the mere removal of a noxious element. At other times it is the preemptive use of anesthesia, while at others it is the prudent use of appropriate analgesia. Pain of course may underly serious disease, and before embarking on therapy it behoves the caregiver to be aware of life threatening diseases that if they go undiagnosed, and furthermore masked by premature treatment, a fatal outcome is possible.
The creation of a comprehensive pain treatment plan requires a thorough understanding of the pain source, the pharmacological properties of any proposed analgesics and the patient?s background. Once implemented, social support and close patient surveillance will optimize outcomes and minimize risk. One must remember however that across all patient cohorts pain is both a necessary evil and a valuable tool requiring medical knowledge, measured inquisitiveness and empathy. As clinicians navigate through the various pain modules of this site and gain practical experience, it will become apparent that pain and its treatment are rarely the same among patients. This imperfection is one of many in medicine but it underscores the artistry necessary to practice the discipline.
References
Peter Lierz, Stefan Punsmann: Opioids In Pain Therapy. Pain, Symptom Control and Palliative Care. 2000. Volume 1 Number 1.
Freye E, Latasch L. Klinik fur Gefasschirurgie und Nierentransplantation, Heinrich-Heine-Universitatsklinik, Dusseldorf. Development of opioid tolerance – molecular mechanisms and clinical consequences. Anasthesiol Intensivmed Notfallmed Schmerzther. 2003 Jan;38(1):14-26.
Boogaerts J, Lafont N. Mechanism of action and clincal use of opioids administered by the peripheral perineural route. Cahiers d’Anesthesiologie. 1991;39:91-9.
Raj P, Practical Management of Pain, Third Edition, Mosby Inc. Copyright 2000
http://www.westmeadanaesthesia.org/pain/acutepainreview.html#SlideFrame_1
http://www.iasp-pain.org/AM/Template.cfm?Section=Home&Template=/CM/ContentDisplay.cfm&ContentID=1960
http://www.medscape.com/viewarticle/456762_10 Clifford Woolf Cox 2 prostaglandins